Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Many targeted synthetic DMARD (tsDMARD) and biological DMARD (bDMARD) were recently approved for treatment of active psoriatic arthritis (PsA), but their comparative efficacy and safety remain unknown. This study aims to compare the efficacy and safety of recently generated tsDMARD and novel bDMARD in active PsA.
Methods:
Randomized clinical trials (RCT) evaluating the efficacy and safety of tsDMARD including tofacitinib (TOF) and apremilast (APR), and bDMARD such as ustekinumab (UST), secukinumab (SEC), ixekizumab (IXE), clazakizumab (CLA), and abatacept (ABA) for active PsA, who received a diagnosis of PsA at least 6 months previously, fulfilled the Classification Criteria for Psoriatic Arthritis, and had active plaque psoriasis and active arthritis (¡Ý3 swollen and ¡Ý3 tender or painful joints) at screening, were identified by comprehensive systemic literature review. Pairwise meta-analysis and Network meta-analysis (NMA) using a random effects model were performed to estimate pooled odds ratios (ORs) and 95% confidence intervals (CIs) of attaining 20% or 50% improvement in ACR criteria (ACR20 or ACR50) and 75% improvement in psoriasis area and severity index (PASI75), any adverse events (AE) and serious adverse events (SAE) across trials.
Results:
We deemed 15 RCT eligible, including 6004 participants and 17 treatments. For efficacy, pairwise meta-analysis showed that all treatments except CLA 200mg/25mg monthly were superior to placebo in achieving ACR20, all except CLA 200mg monthly and ABA 125mg weekly were superior in achieving ACR50, and TOF, APR 30/20mg twice daily, UST, SEC 300mg/150mg monthly and IXE were superior in achieving PASI75. For safety, TOF 10mg twice daily, APR 30mg/20mg twice daily, and IXE were more likely to have any AE, but no significant differences were seen for pooled SAE among the various treatments. NMA showed SEC 300mg monthly to be more efficacious in achieving ACR20 and ACR50, whereas UST 90mg 12 weekly had the highest efficacy in achieving PASI75. SEC 75mg monthly and UST 90mg 12 weekly had the lowest probability of pooled AE and SAE, respectively. Regarding the overall efficacy in both articular and cutaneous aspects and overall risk-benefit profile, SEC and UST ranked higher than other interventions.
Conclusion:
Secukinumab and Ustekinumab may be the safest and most efficacious (for arthritis and psoriasis, respectively) for active PsA among the new targeted synthetic and biological DMARD.
Figure 1: Clustered ranking plot for efficacy and safety
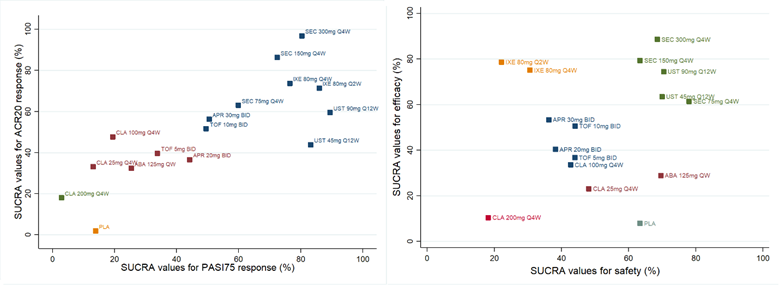
Each color represents a group of treatments that belong to the same cluster. Treatments lying in the upper right corner are more effective or/and safer than the other treatments. (Efficacy= 50% ACR20 response and 50% PASI75 response; Safety= 50% AE and 50% SAE). QD: daily; BID: twice daily; QW: weekly; Q2W: every 2 weeks; Q4W: every 4 weeks; Q12W: every 12 weeks; PLA: placebo.
To cite this abstract in AMA style:
Lu C, Wallace B, Fu W, Liu Y. Efficacy and Safety of Novel Targeted Synthetic DMARD and Biological DMARD in Active Psoriatic Arthritis: A Systematic Review, Meta-Analysis, and Network Meta-Analysis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-novel-targeted-synthetic-dmard-and-biological-dmard-in-active-psoriatic-arthritis-a-systematic-review-meta-analysis-and-network-meta-analysis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-novel-targeted-synthetic-dmard-and-biological-dmard-in-active-psoriatic-arthritis-a-systematic-review-meta-analysis-and-network-meta-analysis/
