Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: As part of the 2016 update of the ASAS/EULAR recommendations for the management of axSpA, we performed a systematic literature review (SLR) to assess the efficacy and safety of non-biological therapy in patients with axSpA published since the last SLR in 2009.
Methods: This SLR was performed in Medline, Embase and Cochrane databases (2009-2016) and also in 2014-2015 EULAR and ACR abstracts and included randomized controlled trials (RCT), clinical controlled trials (for efficacy and safety) and observational studies with a comparator (for safety). Any non-biological drugs or non-pharmacological therapies were eligible. All important efficacy and safety outcomes were analysed. Risk of bias was assessed according to the Cochrane and Hayden tools. Due to heterogeneity no meta-analyses were performed. When possible the Cohen´s Effect size was calculated for non-pharmacological treatments.
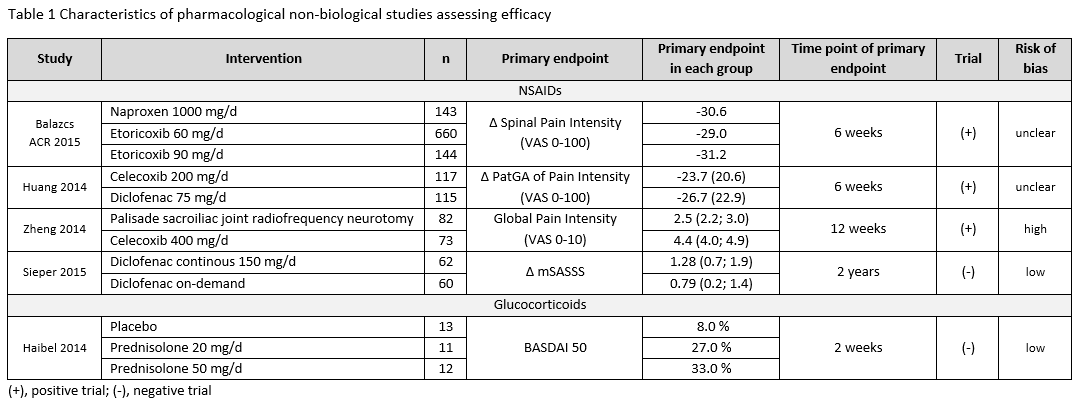
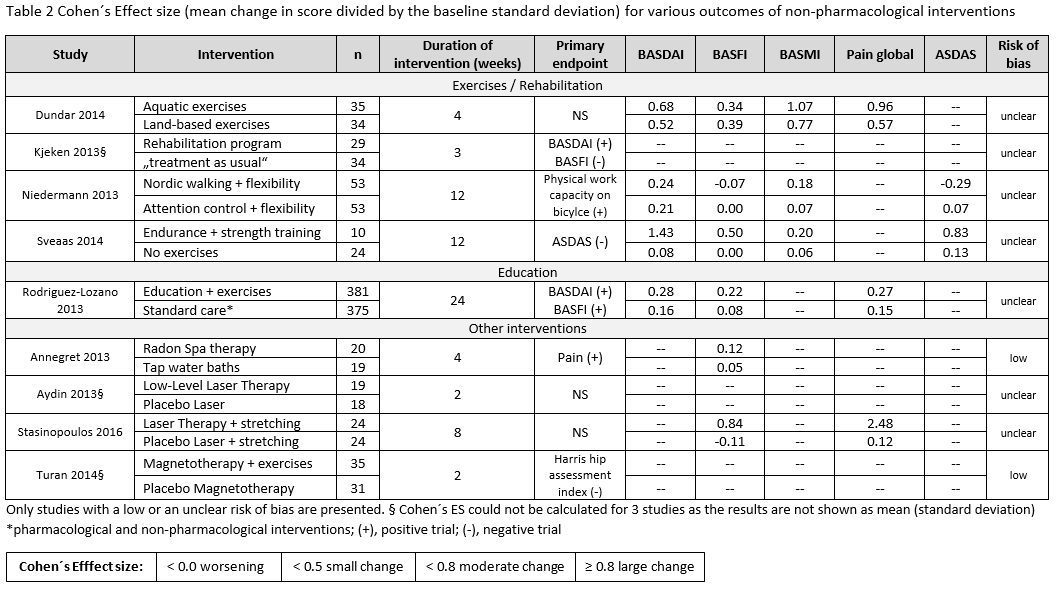
Results: Of 10,244 articles and 428 conference abstracts, we included 42 papers and 3 abstracts [non-biological drugs (table 1): 15 trials, 1 Cochrane review; non-pharmacological therapies: 29 trials]. A Cochrane review strengthened the efficacy and safety of NSAIDs for the treatment of axSpA. This was confirmed by two additional studies (table 1). NSAIDs used continuously compared with on-demand did not reduce the mSASSS mean change over 2 years in AS-patients with normal CRP (≤5mg/l) [1 negative RCT (0.9 vs. 0.8; p=0.62)]; while for patients with high CRP an unclear effect was found [1 positive RCT (0.2 vs. 1.7; p=0.003), 1 negative RCT (1.68 vs. 0.96; p=0.28)]. Based on 4 observational studies, there were no new findings on safety of NSAIDs. No efficacy was shown for two different doses of systemic glucocorticoids in short-term treatment (BASDAI50 week 2: 8% PBO; 27% prednisolone (PDN) 20mg; 33% PDN 50mg). No new trials on conventional synthetic (cs) DMARDs were found. Studies on non-pharmacological therapies were very heterogenous regarding type of therapy, study duration, group size and outcome parameters. All studies included patients fulfilling the mNY-criteria, only one followed the ASAS criteria. Overall they show that regular exercises can improve disease activity, function and spinal mobility in axSpA, but mostly with small improvements (table 2).
Conclusion: Efficacy and safety of NSAIDs in axSpA is confirmed. No data is found on csDMARDs. Glucocorticoids did not demonstrate efficacy in axSpA. Regular exercises can improve outcomes, but with modest effects.
To cite this abstract in AMA style:
Regel A, Sepriano A, Baraliakos X, van der Heijde D, Braun J, Landewé R, van Den Bosch F, Ramiro S. Efficacy and Safety of Non-Biological Therapy (Non-Biological Drugs and Non-Pharmacological Interventions): A Systematic Literature Review for the 2016 Update of the ASAS/EULAR Recommendations for the Management of Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-non-biological-therapy-non-biological-drugs-and-non-pharmacological-interventions-a-systematic-literature-review-for-the-2016-update-of-the-asaseular-recommendations-for-the/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-non-biological-therapy-non-biological-drugs-and-non-pharmacological-interventions-a-systematic-literature-review-for-the-2016-update-of-the-asaseular-recommendations-for-the/