Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Neihulizumab is a novel immune checkpoint agonistic antibody that binds to human CD162 (PSGL-1), thereby preferentially inducing apoptosis in late stage activated T cells. It is being tested in putative T-cell mediated inflammatory diseases including ulcerative colitis and graft versus host disease. To assess the efficacy and safety of Neihulizumab in patients with active psoriatic arthritis (PsA) in an open-label 24-week Phase II POC study.
Methods: Twenty (20) patients with active PsA fulfilling Classification Criteria for Psoriatic Arthritis (CASPAR) were treated with 3 weekly doses plus 4 bi-weekly doses of 9 mg/kg IV Neihulizumab with follow-up at weeks 12, 16, 20, and 24. The primary endpoint was the proportion of patients achieving an ACR 20 response at Week 12. Safety was assessed throughout the study period.
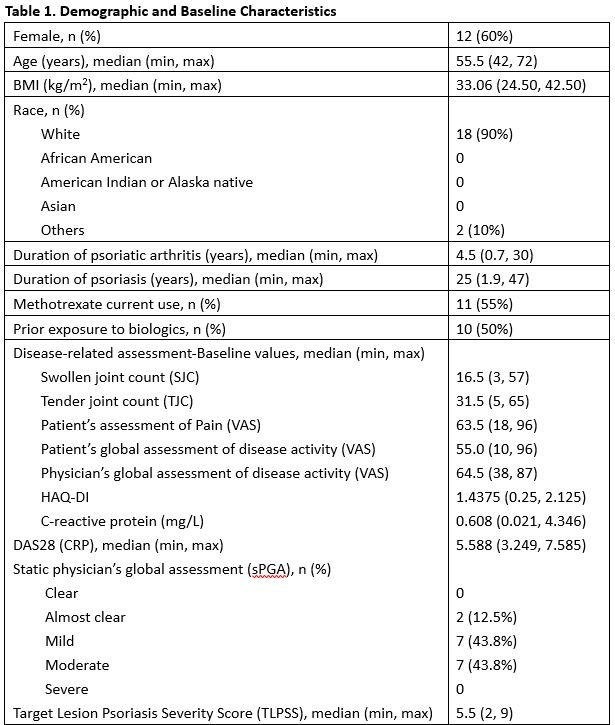
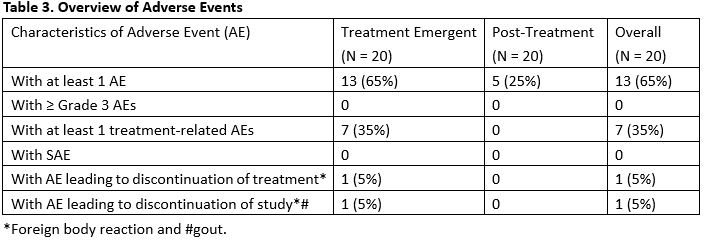
Results: Twenty patients (20) were enrolled. The majority of the patients were female (12/20, 60.0%) and white (18/20, 90.0%). The median age was 55.5(42-72) years. The median duration of psoriatic arthritis was 4.5 (0.7-30) years. The median duration of psoriasis was 25 (1.9-47) years. Forty (40.0) % (8/20) of patients achieved ACR20 responder status at Week 12 in ITT population by Non-Responder Imputation. At week 12 the ACR50 and 70 response rates were 30%, and 10%, respectively. Analysis by DAS28(CRP) showed concordant result. Durability of ACR20/50/70 was maintained through week 24 for at least 50% of responders after the last treatment at Week 10. Neihulizumab treatment was well tolerated with no deaths, no serious AEs, and no severe AEs observed. The most frequent TEAEs overall (including the treatment period and follow-up period) were urinary tract infection (15.0%), psoriatic arthropathy (15.0%), headache (10.0%), sinus congestion (10.0%), and hematoma (10.0%).
Conclusion: Overall treatment of Neihulizumab was well tolerated in this study. Improvement was seen in efficacy parameters, suggesting there may be clinical utility with this novel agent for the treatment of psoriatic arthritis. Controlled studies are indicated
ClinicalTrials.gov Identifier: NCT02267642
To cite this abstract in AMA style:
Cohen S, Fiechtner J, Mease P, Kaine J, Kavanaugh A, Cheng Y, Chou C, Cheng T, Lin S, Genovese M. Efficacy and Safety of Neihulizumab (AbGn-168H) in Patients with Active Psoriatic Arthritis: 24-week Results from a Phase II Open Label Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-neihulizumab-abgn-168h-in-patients-with-active-psoriatic-arthritis-24-week-results-from-a-phase-ii-open-label-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-neihulizumab-abgn-168h-in-patients-with-active-psoriatic-arthritis-24-week-results-from-a-phase-ii-open-label-study/