Session Information
Date: Tuesday, November 10, 2015
Title: Vasculitis III
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose:
Polymyalgia rheumatica (PMR) is characterized
by persisting proximal pain and morning stiffness of the neck, shoulder and hip
girdles of 2 weeks’ duration,
an acute-phase response, and a rapid clinical response to glucocorticoids. Modified
release (MR) prednisone improves oral prednisone treatment strategies, at least
in RA, by adapting glucocorticoid release to endogenous cortisol rhythms and
symptom severity, both of which have their peaks during the early morning
hours. We assessed the efficacy and safety of MR prednisone compared
to immediate release (IR) prednisone in patients with newly diagnosed PMR
previously untreated with glucocorticoids.
Methods:
Patients
meeting the 2012 EULAR/ACR classification criteria for PMR (excluding US) were
randomized to double-blind MR prednisone or IR prednisone 15 mg/day for
4 weeks. MR prednisone/placebo was taken at approx. 10pm and IR prednisone/placebo
was taken between 5am and 9am. Patients recorded duration of morning stiffness
and symptoms of PMR, global pain, shoulder pain and fatigue on visual analog
scales (VAS) in a daily diary. CRP, ESR and IL-6 were measured at study visits.
The primary efficacy endpoint was the percentage of complete responders (CRs, defined
as ≥70% reduction in PMR VAS, duration of morning stiffness and CRP [or
CRP <2 x ULN]) at Week 4, analyzed using a logistic regression model. Non-inferiority
was concluded if the lower limit of the 95% CI for the treatment comparison (MR
vs. IR prednisone) was above -15%.
Results:
The study randomized 62 patients; 66% female,
mean age 69 years. The percentage of CRs at Week 4 was 54% for
MR prednisone and 41% for IR prednisone in the per protocol population (PPP)
(53% and 33%, respectively, in the full analysis population [FAP]). Non‑inferiority of MR vs. IR prednisone
was not proved in the primary analysis on the PPP (N=48; treatment difference:
12.22% in favour of MR prednisone; 95% CI: -15.82%, 40.25%) as the lower 95% CI
was less than ‑15%, but sensitivity analysis on the FAP showed a trend in
favour of MR prednisone (N=62; treatment difference: 15.56%; 95% CI: -9.16%,
40.28%). There was a
clear consistent trend for a stronger effect of MR compared with IR prednisone across
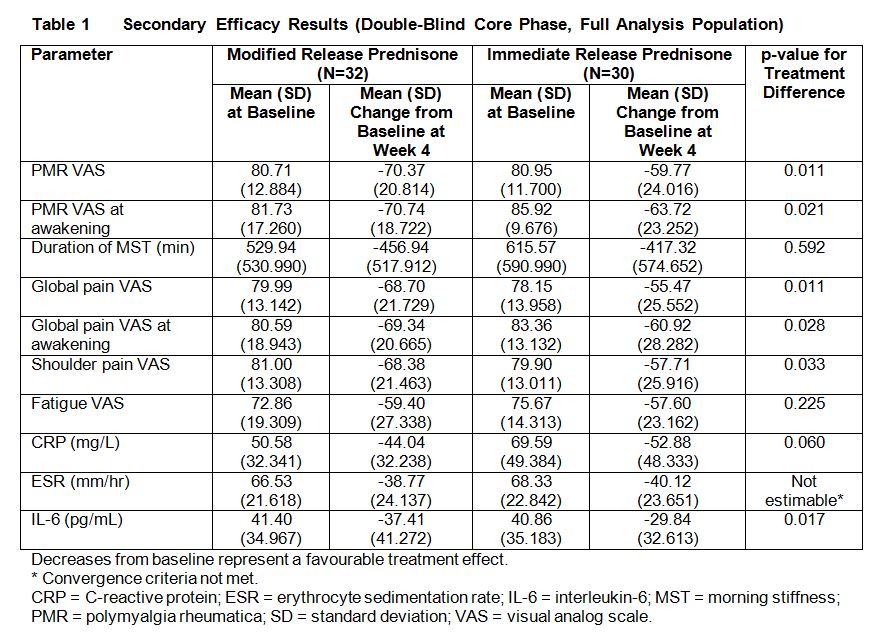
most secondary efficacy endpoints at Week 4 (Table 1), with a discernible
treatment difference observed as early as Week 1. MR prednisone showed a larger
efficacy in reducing IL‑6 levels. The
incidence of treatment-related adverse events was relatively low and the events
reported were generally consistent with the known safety profiles of used doses
of MR and IR prednisone.
Conclusion:
Although
the primary analysis of non-inferiority was not met, the
consistently positive and clinically meaningful results for MR prednisone compared
with IR prednisone observed in this study provide an indication of a beneficial
clinical effect of MR over IR prednisone in patients with PMR, with
improvements observed as early as Week 1.
To cite this abstract in AMA style:
Cutolo M, Hopp M, Liebscher S, Dasgupta B, Buttgereit F. Efficacy and Safety of Modified-Release Prednisone in Patients with Polymyalgia Rheumatica: Results of a Multicenter, Randomized, Active-Controlled Phase 3 Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-modified-release-prednisone-in-patients-with-polymyalgia-rheumatica-results-of-a-multicenter-randomized-active-controlled-phase-3-study/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-modified-release-prednisone-in-patients-with-polymyalgia-rheumatica-results-of-a-multicenter-randomized-active-controlled-phase-3-study/