Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) are a key treatment modality for major organ involvement, one of the main contributors to morbidity and mortality, as well as for resistant mucocutaneous and articular manifestations that significantly impair quality of life in Behçet’s Syndrome (BS). In cases of inadequate response, and with emerging evidence on the involvement of multifactorial immune mediators and activation of the Janus kinase (JAK) signaling pathway in BS, JAK inhibitors (JAKi) have been introduced through pilot studies and case reports. We aimed to investigate the efficacy and safety profile of JAKi through a systematic literature review.
Methods: The systematic literature review was registered in PROSPERO under the identification number CRD420251000172. All articles published in PubMed, EMBASE, and the Cochrane Library up to March 6, 2025, that reported the use of JAKi in patients with BS and provided treatment outcomes were included. The search was conducted using the keywords: “Behçet AND (JAK OR tofacitinib OR baricitinib OR upadacitinib OR filgotinib OR Janus).” Two independent investigators (BS and SNE) screened 306 articles, and a total of 18 articles, reporting on 91 patients, along with one case report from our center, were included. Four patients (4.3%) had a prior history of JAKi use.
Results: Among the included patients, 16 (17.4%) received upadacitinib, 49 (53.3%) received tofacitinib, and 31 (33.7%) received baricitinib. The primary indication for initiating JAKi therapy was uveitis in 18 patients (19.6%), vascular involvement in 22 (23.9%), and gastrointestinal involvement in 46 (50%). The remaining 5 patients received JAK inhibitors due to refractory mucocutaneous and/or articular involvement. Nearly all patients had previously failed conventional treatments and 57.1% at least one anti-TNF monoclonal antibody. The remission rates were 82.6% and 50% for gastrointestinal involvement in terms of clinical and endoscopic remission, respectively; 83.3% for ocular involvement; and 90.9% for vascular involvement. The relapse rates were 2.2% for gastrointestinal and 16.7% for ocular involvement (Table 1). Serious adverse events were reported in 6 patients (6.5%), including two cases of anemia, one of pneumonia, and three cases of herpes zoster. No thrombotic events were reported in any patient.
Conclusion: JAK inhibitors are promising targeted synthetic DMARDs for patients with BS who show inadequate response to TNF inhibitors, particularly in cases with major organ involvement. However, randomized controlled trials are needed to establish their true efficacy and safety profile.
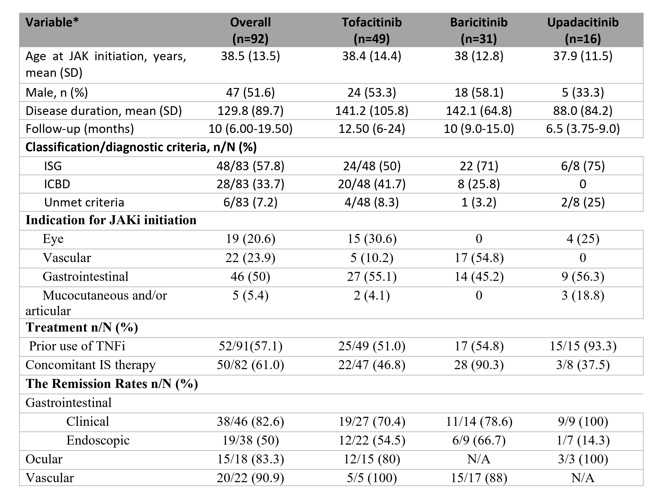 Table 1. Demographics and clinical characteristics of patients with BS who used JAKi identified by systematic review including our case
Table 1. Demographics and clinical characteristics of patients with BS who used JAKi identified by systematic review including our case
To cite this abstract in AMA style:
Sulu B, Esatoglu S, Hatemi I, Celik A, Hatemi G. Efficacy and Safety of JAK Inhibitors in Behçet’s Syndrome: A Systematic Literature Review [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-jak-inhibitors-in-behcets-syndrome-a-systematic-literature-review/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-jak-inhibitors-in-behcets-syndrome-a-systematic-literature-review/
