Session Information
Session Type: Poster Session
Session Time: 10:30AM-12:30PM
Background/Purpose: PsA is a chronic, systemic, inflammatory musculoskeletal disease in which dysregulated IL-17A activity plays a pivotal role in disease pathogenesis. Izokibep (IZO) is an Affibody® molecule (small protein therapeutic; 18.6 kDa) designed to inhibit IL-17A with high potency through tight selective binding. In a phase 2b/3 study in patients (pts) with active PsA, IZO was well tolerated and demonstrated improved efficacy vs placebo (PBO) across multiple disease domains including the study primary endpoint of ACR50 at week (wk) 16. Here, we report the efficacy and safety of IZO from the phase 2b/3 study through wk 52.
Methods: In this study (NCT05623345; Study 22104), eligible pts had adult-onset active PsA (duration ≥6 months and ≥3 tender/swollen joints) and an inadequate response, intolerance, or contraindication to an NSAID, conventional synthetic DMARD, and/or TNF inhibitor. During the 16-wk PBO-controlled treatment period, pts were equally randomized to IZO 160 mg every 2 wks (Q2W), IZO 160 mg every wk (QW), or PBO QW. At wk 16, pts originally randomized to IZO kept receiving IZO per their original dose and schedule; pts receiving placebo were switched to IZO 160 mg QW. Wk 52 results are reported.
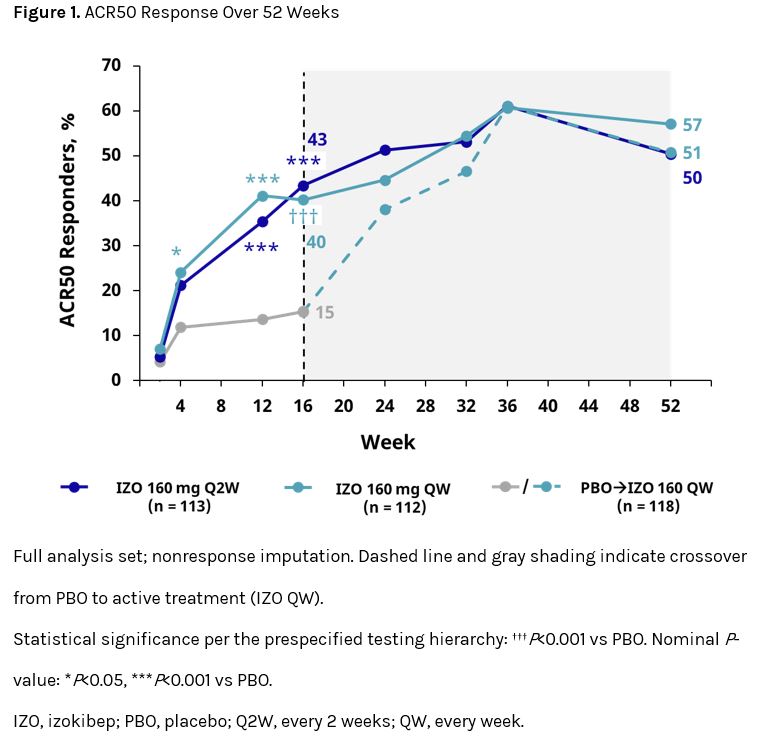
Results: A total of 343 pts were included (IZO Q2W, n = 113; IZO QW, n = 112; PBO→IZO QW, n = 118). The mean (SD) age was 49.5 (13.3), 51.8 (12.2), and 52.6 (11.7) years, BMI was 30.5 (6.6), 29.1 (5.9), and 29.7 (6.0) kg/m2, and time since diagnosis was 6.2 (6.8), 6.9 (8.0), and 7.1 (6.9) years for IZO Q2W, IZO QW, and PBO→IZO QW, respectively; 59%, 57%, and 43% of pts were male. Baseline (BL) disease characteristics were generally similar across groups. After wk 16, continued improvement was seen for pts randomized to either IZO group and rapid improvement was observed in pts following crossover from PBO (Figure 1); by wk 52, approximately half of pts achieved ACR50 (IZO Q2W, 50%; IZO QW, 57%; PBO→IZO QW, 51%). Notable percentages of pts in all groups also achieved the high-hurdle endpoints of ACR70 (36%, 42%, 42%), PASI90 (63%, 69%, 65%), PASI100 (55%, 64%, 58%), and minimal disease activity (MDA; 47%, 52%, 47%) at wk 52 (Table 1). Improvements in quality of life measures were also observed across groups (Table 1). Overall, more than half of pts with BL enthesitis achieved resolution at wk 52 (Table 1). Treatment-emergent adverse events (TEAEs) occurred in 81%, 88%, and 82% of pts receiving IZO Q2W, IZO QW, and PBO→IZO QW, respectively, through wk 52; the most common TEAEs were injection-site erythema and pruritus and nasopharyngitis. The majority of TEAEs were mild to moderate in severity, with serious TEAEs reported at low rates (IZO Q2W 7%; IZO QW 4%; PBO→IZO QW 4%). Rates of ulcerative colitis (0%, 1%, 0%) and oral candidiasis (1%, 1%, 0%) were also low. There were no deaths or reports of suicidal ideation.
Conclusion: IZO demonstrated durable efficacy across several measures of PsA disease activity over 52 wks. Substantial percentages of pts originally randomized to IZO or switching from PBO to IZO achieved ACR50/70, PASI 90/100, and MDA by the end of the study. IZO treatment was well tolerated, with a safety profile generally consistent with that of legacy IL-17A inhibitors.
To cite this abstract in AMA style:
Mease P, Behrens F, Kivitz A, Drescher E, Klimiuk P, Sofen H, Soloman N, Mpofu S, Frejd F, Taylor P. Efficacy and Safety of Izokibep, a Novel IL-17A Inhibitor, in Patients with Active Psoriatic Arthritis: Week 52 Results from a Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase 2b/3 Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-izokibep-a-novel-il-17a-inhibitor-in-patients-with-active-psoriatic-arthritis-week-52-results-from-a-randomized-double-blind-placebo-controlled-multicenter-phase-2b-3-stu/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-izokibep-a-novel-il-17a-inhibitor-in-patients-with-active-psoriatic-arthritis-week-52-results-from-a-randomized-double-blind-placebo-controlled-multicenter-phase-2b-3-stu/

.jpg)