Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: GALAXI 2 & 3 (G2 & G3) are identical 48-week, randomized, double-blind, double-dummy, placebo (PBO)- and active- comparator (ustekinumab; UST) treat-through registrational trials assessing the efficacy and safety of guselkumab (GUS), a dual-acting IL-23p19 subunit antagonist, in patients with Crohn’s disease (CD).
Methods: Patients with moderately to severely active CD (Crohn’s Disease Activity Index [CDAI] 220–450 + mean daily SF >3 or AP >1), Simple Endoscopic Score [SES]-CD≥6 (≥4 for isolated ileal disease), and inadequate response or intolerance to conventional or biologic (BIO-ir) therapy were eligible. Randomization was stratified by CDAI (≤300 or >300), SES-CD (≤12 or >12), BIO-ir status, and corticosteroid (CS) use with patients assigned 2:2:2:1 to GUS 200 mg intravenous (IV) every 4 weeks (q4w) (x3)→200 mg subcutaneous (SC) q4w, GUS 200 mg IV q4w (x3)→100 mg SC q8w, UST ~6 mg/kg IV (x1)→90 mg SC q8w, or PBO. PBO patients not in clinical response at week 12 (W12) switched to UST IV→90 mg SC q8w. In each trial, the composite co-primary endpoints were clinical response at W12 + clinical remission at W48 and clinical response at W12 + endoscopic response at W48, comparing each GUS regimen to PBO. Primary and major secondary endpoints were multiplicity controlled; treatment differences were analyzed based on the common risk difference test. Pooled analyses of GUS vs UST were prespecified major secondary endpoints.
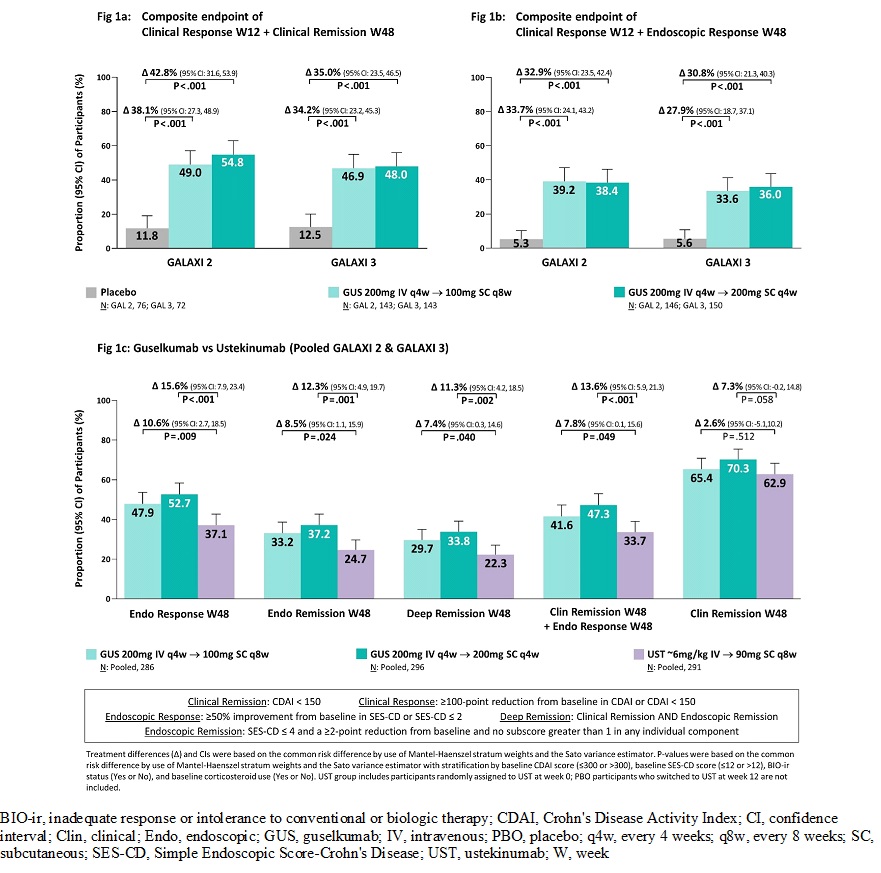
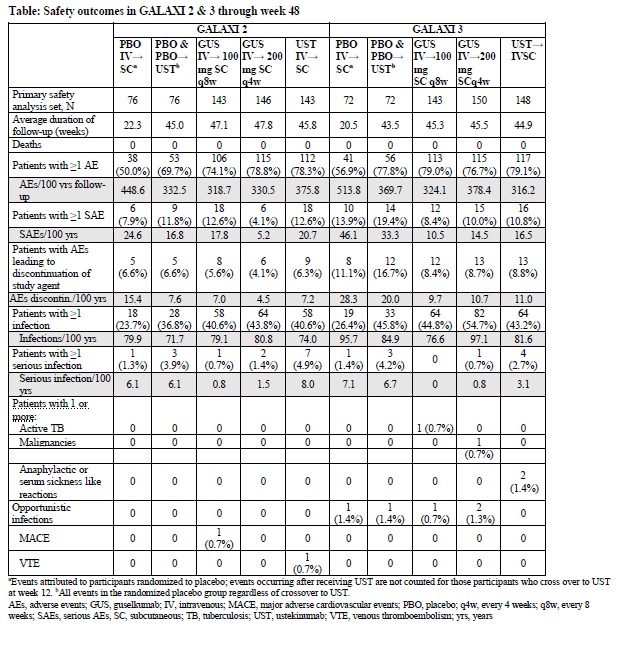
Results: The primary analysis sets in G2 & G3 included 508 and 513 patients, respectively. Baseline characteristics were similar across trials: CDAI (G2: 295.0, G3: 294.7), SES-CD (13.1, 12.8), BIO-ir history (52.8%, 51.9%), oral CS use (37.4%, 36.1%). Patients receiving GUS 200 mg IV had significantly greater (P< .001) rates at W12 vs PBO of clinical remission (G2: 47.1% vs 22.4%, ∆25.1%; G3: 47.1% vs 15.3%, ∆31.2%) and endoscopic response (G2: 37.7% vs 10.5%, ∆27.7%; G3: 36.2% vs 13.9%, ∆22.1%). In both trials, significantly greater (P< .001) proportions of patients receiving GUS 100 mg SC q8w or 200 mg SC q4w achieved the co-primary endpoints relative to PBO (Fig 1a & 1b). In the W48 pooled analyses (Fig 1c) both GUS doses demonstrated superiority to UST for endoscopic response (GUS 100 mg SC q8w vs UST: ∆10.6%, GUS 200 mg SC q4w vs UST: ∆15.6%), endoscopic remission (∆8.5%, ∆12.3%), deep remission (∆7.4%, ∆11.3%), and clinical remission + endoscopic response (∆7.8%, ∆13.6%). Through W48, the proportions of GUS patients with ≥1 adverse events (AEs), ≥1 serious AEs, AEs leading to discontinuation, and infections were similar to PBO and UST (Table). Overall, the proportions of patients with serious infections and AEs of interest were low and similar between GUS and UST.
Conclusion: In the double-blind GALAXI 2 & 3 trials, GUS was statistically superior to PBO for short- and long-term clinical and endoscopic endpoints. Both GUS doses were statistically superior to UST at W48 across multiple major secondary endpoints in the pooled analyses. Safety data for both GUS regimens in patients with CD were consistent with the known safety profile of GUS in approved indications.
To cite this abstract in AMA style:
Panaccione R, Danese S, Feagan B, D’Haens G, Afzali A, Reinisch W, Panés J, Rubin D, Andrews J, Hisamatsu T, Terry N, Salese L, Rampelbergh R, Frustaci M, Yang Z, Johanns J, Wan K, Yee J, Sands B. Efficacy And Safety of Guselkumab Therapy in Patients With Moderately to Severely Active Crohn’s Disease: Results of the Galaxi 2 & 3 Phase 3 Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-guselkumab-therapy-in-patients-with-moderately-to-severely-active-crohns-disease-results-of-the-galaxi-2-3-phase-3-studies/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-guselkumab-therapy-in-patients-with-moderately-to-severely-active-crohns-disease-results-of-the-galaxi-2-3-phase-3-studies/