Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Bruton’s tyrosine kinase (BTK) is involved in signalling pathways known to be important to the pathogenesis of systemic lupus erythematosus (SLE). Evobrutinib is a highly-selective, oral BTK inhibitor. This global Phase IIb randomized, double-blind, placebo-controlled study evaluated the efficacy, dose response and safety of evobrutinib compared to placebo in adult patients with active autoantibody-positive SLE who received standard of care (SoC) therapy.
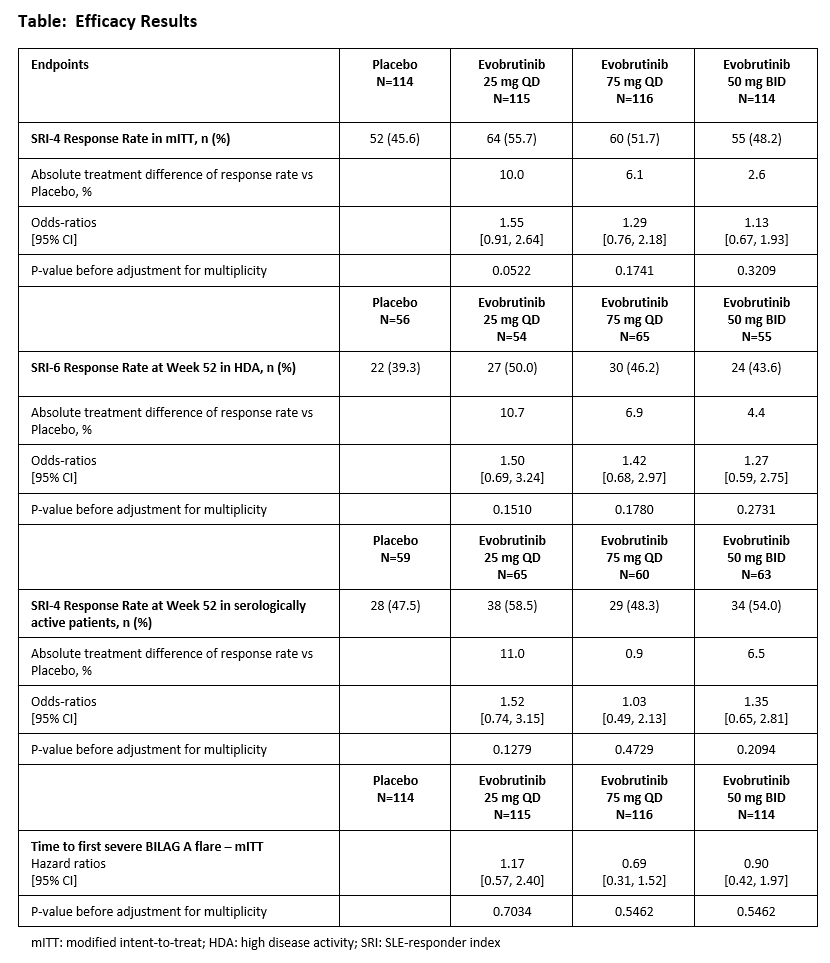
Methods: Patients diagnosed with SLE by the SLICC or ACR classification criteria ≥6 months before screening, who had a SLEDAI-2K total score ≥6 at screening, were autoantibody-positive, and on stable SLE SoC background therapy; were randomized 1:1:1:1 to oral evobrutinib 25 mg once-daily (QD), 75 mg QD, or 50 mg twice-daily (BID), or placebo. Active or severe central nervous system manifestations were exclusionary. The two primary endpoints were the SLE-Responder Index (SRI)-4 at Week 52 in all patients and the SRI-6 response at Week 52 in the subgroup of patients with High Disease Activity (defined as SLEDAI-2K total score ≥10 at screening). Primary safety endpoints included the incidence and severity of treatment-emergent adverse events (TEAEs), and key secondary endpoints were SRI-4 response in the serologically active subgroup, defined as patients with positive anti-dsDNA antibodies and/or low complement levels at screening, and time to first new severe (BILAG A) flare during treatment.
Results: A total of 469 patients were randomized for the study’s primary analysis. Age at baseline was 40.7 years ±12.3 (mean ±SD) and 95% were female. Baseline disease characteristics were generally balanced across treatment groups. Median disease duration ranged from 51.6 to 69.2 months across treatment groups. Neither primary endpoints, SRI-4 response at Week 52 or SRI-6 response in patients with High Disease Activity, were met (Table). There was no clinically meaningful treatment difference for SRI-4 response in the serologically active subpopulation (Table). No treatment effect was observed for time to first severe flare. Serious adverse events were infrequently reported in all groups, 8.5% in placebo versus 9.4% in the evobrutinib groups combined. Infections and infestations were the most common treatment-emergent adverse event (TEAE), 42.7% for placebo versus 58.0% for evobrutinib groups combined. Overall, there was no dose effect in TEAEs or SAEs.
Conclusion: This Phase IIb dose-ranging study in SLE showed no treatment effect of evobrutinib versus placebo at any dose. Evobrutinib was well tolerated across the overall and high disease activity subgroup populations, and there was no dose effect observed for any adverse events. These results, combined with the negative results of the fenebrutinib SLE study, suggest that BTK inhibition is not an effective therapeutic intervention for patients with SLE.
To cite this abstract in AMA style:
Wallace D, Dörner T, Pisetsky D, Sanchez-Guerrero F, Kao A, Parsons-Rich D, Patel A, Zima Y, Le Bolay C, Thangavelu K, Dall'Era M. Efficacy and Safety of Evobrutinib (M2951) in Adult Patients with Systemic Lupus Erythematosus Who Received Standard of Care Therapy: A Phase II, Randomized, Double-blind, Placebo-controlled Dose Ranging Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-evobrutinib-m2951-in-adult-patients-with-systemic-lupus-erythematosus-who-received-standard-of-care-therapy-a-phase-ii-randomized-double-blind-placebo-controlled-dose-rang/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-evobrutinib-m2951-in-adult-patients-with-systemic-lupus-erythematosus-who-received-standard-of-care-therapy-a-phase-ii-randomized-double-blind-placebo-controlled-dose-rang/