Session Information
Date: Sunday, November 13, 2022
Title: Plenary II
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
Background/Purpose: Tyrosine kinase 2 (TYK2) mediates signaling of Type I IFNs, IL-23, and IL-12, key cytokines involved in lupus pathogenesis. Deucravacitinib (DEUC) is an oral, selective, allosteric TYK2 inhibitor with a unique mechanism of action, distinct from Janus kinase (JAK) 1/2/3 inhibitors, and has shown efficacy in psoriasis and psoriatic arthritis. This analysis assessed the efficacy and safety of DEUC in patients with active SLE.
Methods: This was a 48-week (wk), randomized, double-blind, placebo (PBO)-controlled, phase 2 trial (NCT03252587). Eligible patients met the SLICC criteria, were seropositive (ANA/anti-dsDNA/anti-Sm), and had a SLEDAI-2K score ≥ 6 and ≥ 1 BILAG index A or > 2 BILAG B manifestations from the musculoskeletal or mucocutaneous domain. Patients on standard background medications were randomized 1:1:1:1 to PBO or DEUC (3 mg BID, 6 mg BID, or 12 mg QD). Oral corticosteroid tapering to 7.5 mg/day was required from wks 8-20; further tapering was optional from wks 32-40. The primary endpoint was the proportion of patients achieving SLE responder index (SRI[4]) at wk 32. Key secondary endpoints at wk 48 included SRI(4), BILAG-based Composite Lupus Assessment (BICLA), Lupus Low Disease Activity State (LLDAS), decrease of ≥ 50% from baseline Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI-50), and change from baseline in active (tender and swollen) joint count.
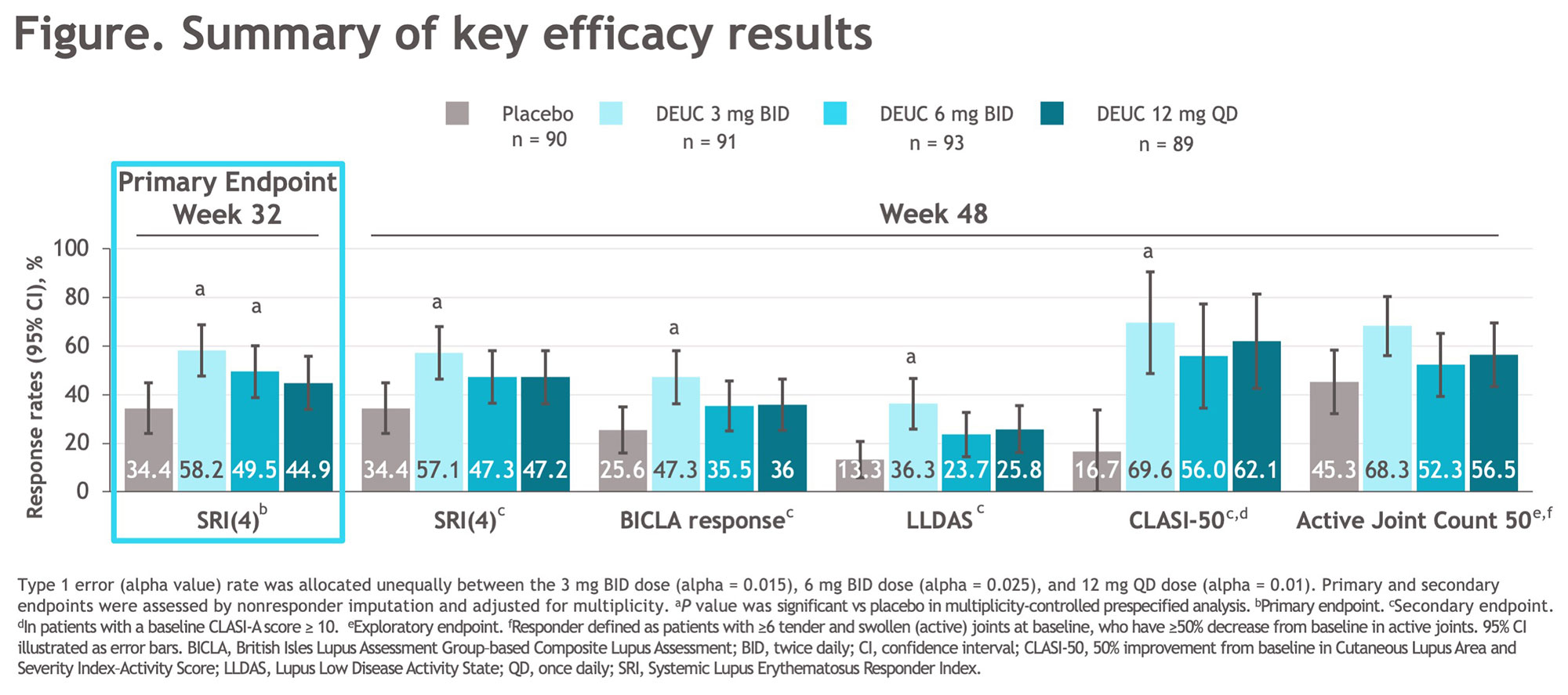
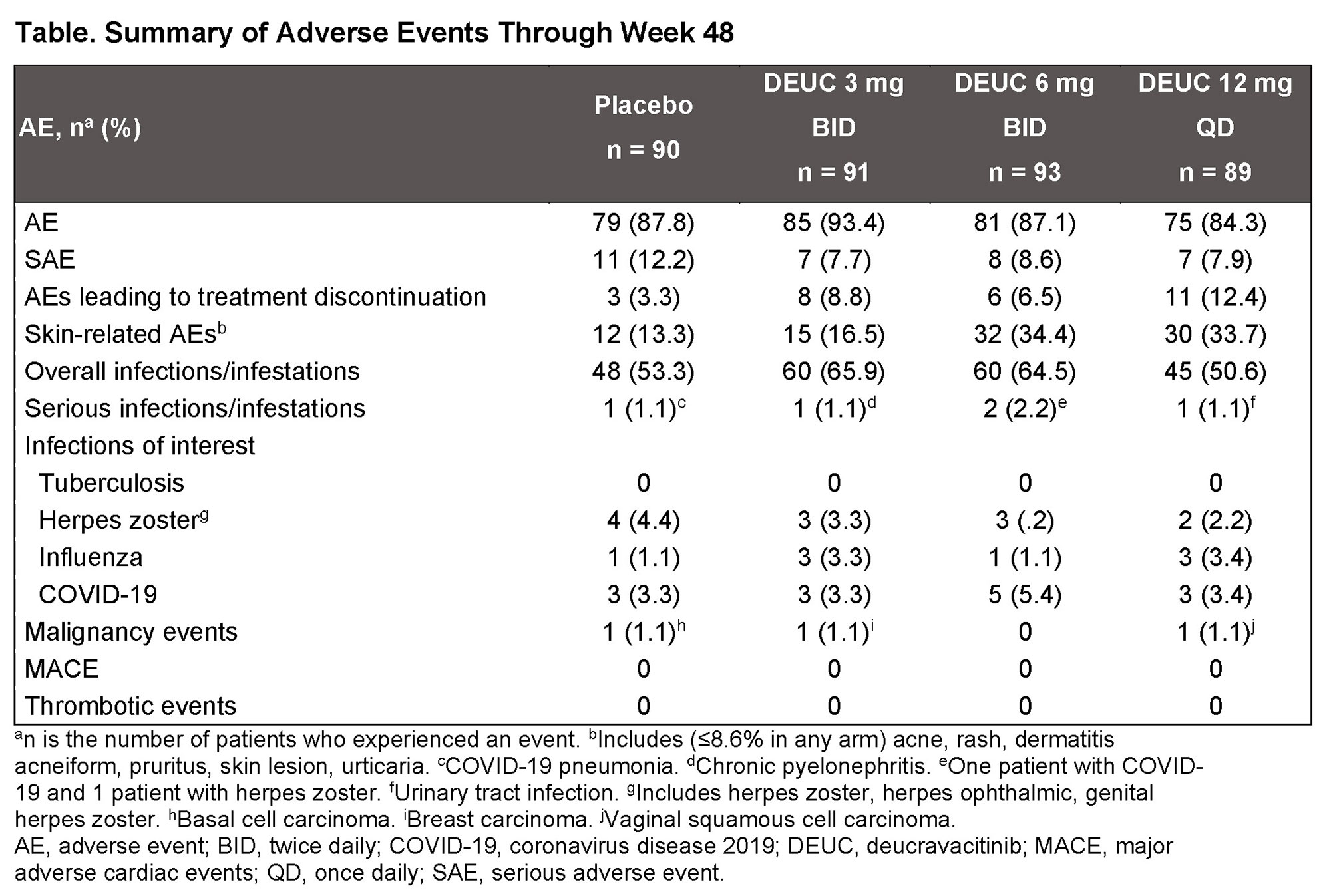
Results: A total of 363 patients were randomized, with baseline demographic and disease characteristics similar across treatment groups. Of randomized patients, 275 (76%) completed 48 wks of treatment. The primary endpoint at wk 32 was met, with significantly greater proportion of patients in DEUC 3 mg BID and 6 mg BID groups vs PBO achieving SRI(4) responses (PBO: 34.4%; DEUC 3 mg BID: 58.2%, P=0.0006; DEUC 6 mg BID: 49.5%, P=0.021; DEUC 12 mg QD: 44.9%, P=0.078). SRI(4) response was sustained across all DEUC groups up to 48 wks (Figure). At wk 48, the DEUC 3 mg BID group demonstrated statistical significance in BICLA, LLDAS, CLASI-50, and active joint count, and the two other DEUC groups demonstrated clinically meaningful differences vs PBO (Figure). Rates of adverse events (AEs), serious AEs, and AEs of interest were similar between DEUC and PBO groups (Table). Most common AEs (≥10%) with DEUC were upper respiratory tract infection, nasopharyngitis, headache, and urinary tract infection. No deaths, major cardiac events, thrombotic events, systemic opportunistic infections, or active tuberculosis occurred. Malignancies were rare with similar rates across all groups. No meaningful abnormalities in mean levels of hematology and chemistry laboratory parameters were observed.
Conclusion: In patients with active SLE, DEUC showed statistically significant and sustained clinical efficacy in SRI(4) responses versus PBO and was well tolerated. All secondary endpoints were achieved or meaningfully improved with DEUC treatment at wk 48, including SRI(4), BICLA, LLDAS, CLASI-50, and change in active joint count. DEUC shows promise as a novel therapy for SLE and warrants further investigation in phase 3 trials.
To cite this abstract in AMA style:
Pike M, Merrill J, Morand E, van Vollenhoven R, Werth V, Hobar C, Delev N, Shah V, Sharkey B, Wegman T, Catlett I, Banerjee S, Singhal S. Efficacy and Safety of Deucravacitinib, an Oral, Selective, Allosteric TYK2 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-deucravacitinib-an-oral-selective-allosteric-tyk2-inhibitor-in-patients-with-active-systemic-lupus-erythematosus-a-phase-2-randomized-double-blind-placebo-controlled-stu/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-deucravacitinib-an-oral-selective-allosteric-tyk2-inhibitor-in-patients-with-active-systemic-lupus-erythematosus-a-phase-2-randomized-double-blind-placebo-controlled-stu/