Session Information
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Dapirolizumab pegol (DZP) is a polyethylene glycol-conjugated Fab’ fragment, which targets CD40 ligand and is in development for the treatment of systemic lupus erythematosus (SLE). The primary objective was to establish a dose-response relationship for DZP using prespecified models. Other objectives were to compare the safety and efficacy of DZP to standard of care (SOC) treatment, and to assess the durability of response after study drug withdrawal.
Methods: This phase 2b study (NCT02804763) consisted of a 24-week double-blind (DB), placebo (PBO)-controlled period (study drug plus SOC), followed by a 24-week observational period (SOC only). Patients were randomized 1:1:1:1 to SOC+PBO or SOC+intravenous DZP (6/24/45 mg/kg) every 4 weeks to Week 24. The primary objective was measured at Week 24, and other objectives throughout the study.
Adults with SLE (SLICC classification criteria) and moderate to severe disease activity (SLEDAI-2K score ≥6 and ≥1 BILAG A or ≥2 BILAG B domain scores), receiving stable doses of corticosteroids (CS; ≤40 mg/day prednisone-equivalent) and/or antimalarials and/or immunosuppressants, were eligible. Patients receiving CS ≥10 mg/day were required to start tapering within 4 weeks of first study drug infusion. Patients with stable lupus nephritis were permitted entry.
Clinical outcomes were analyzed in patients who received ≥1 full dose of study medication and had ≥1 post-baseline efficacy measurement. Safety and immunologic outcomes were analyzed in patients who received ≥1 dose of study medication (any dose).
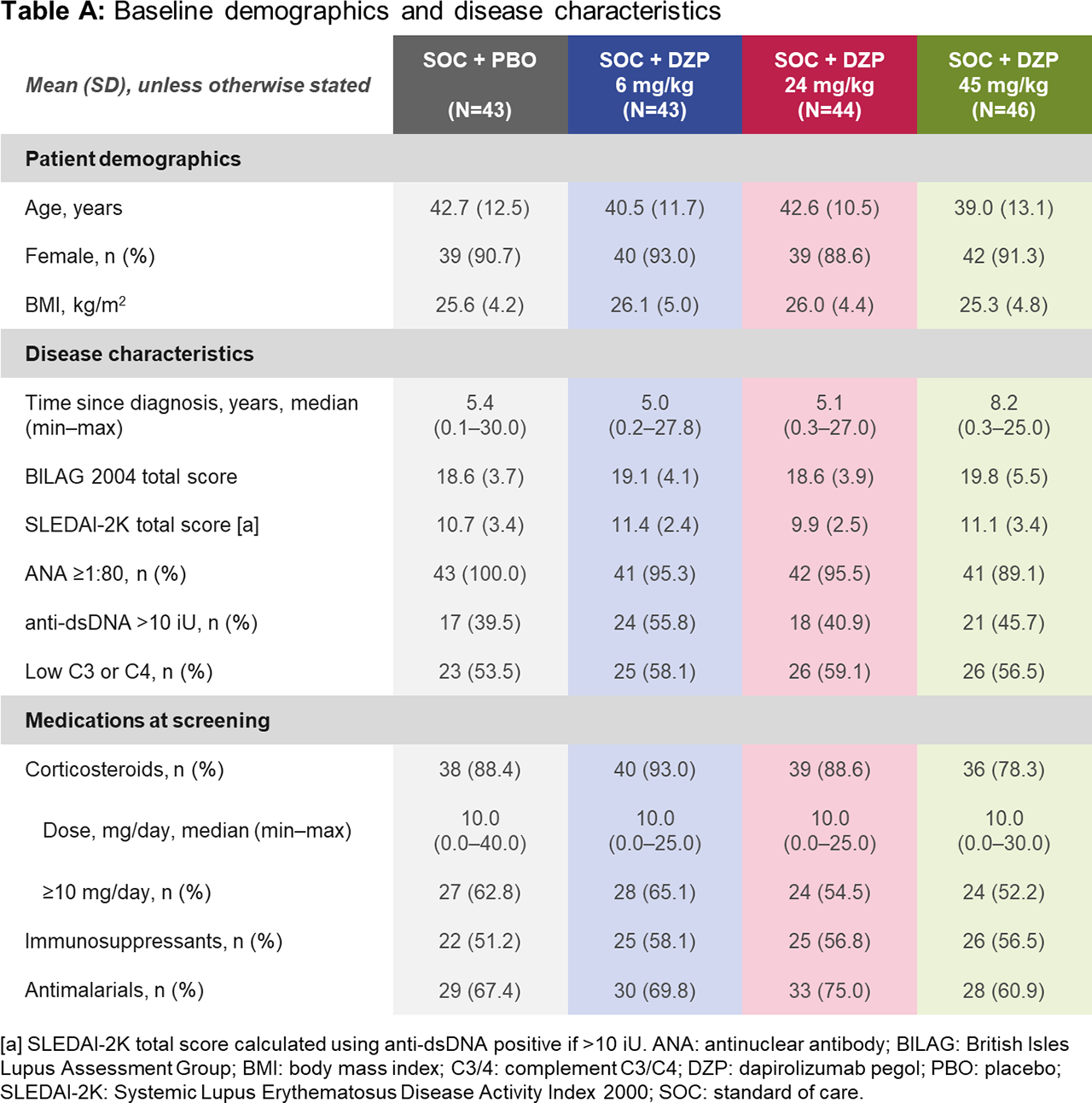
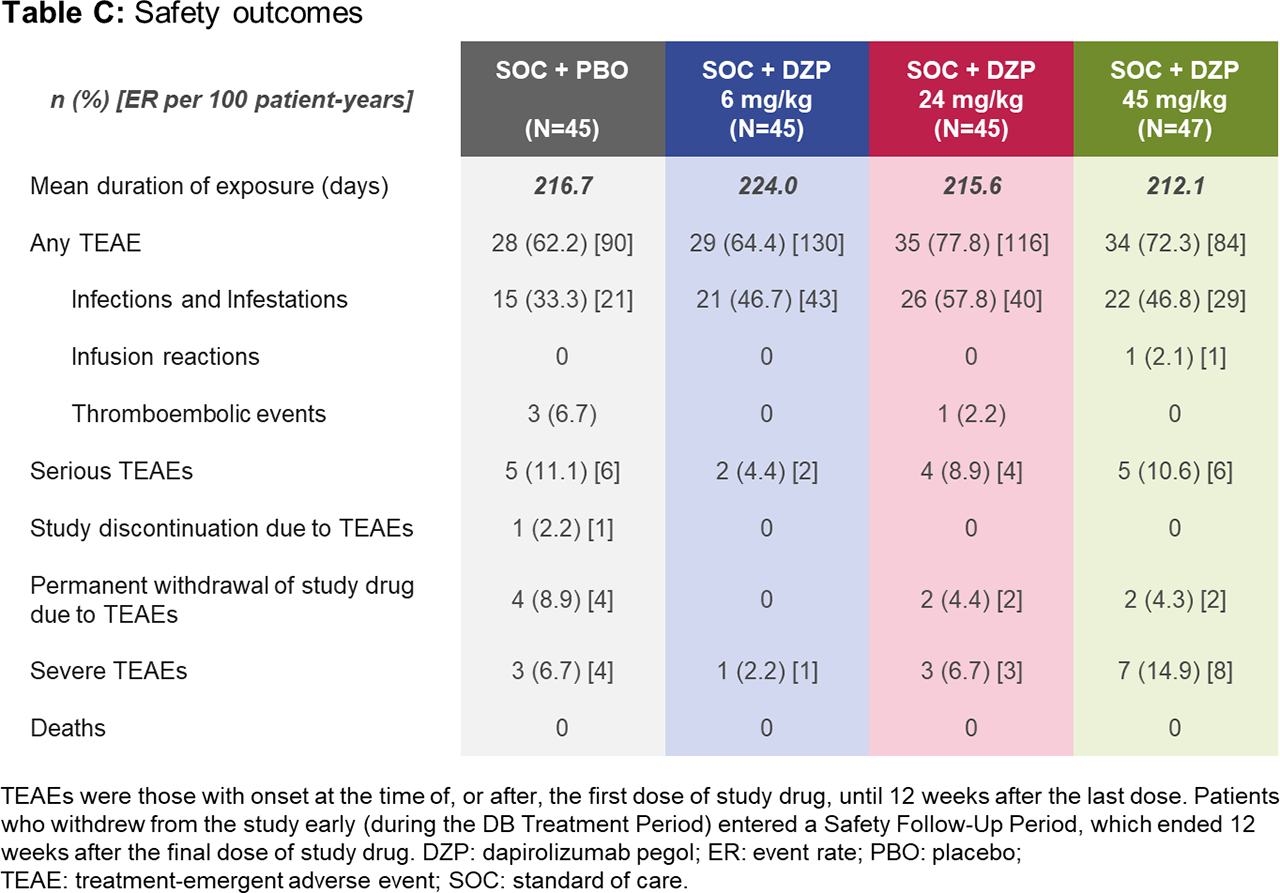
Results: Of 182 randomized patients, 178 (97.8%) completed the DB period to Week 24 (including 167 [91.8%] on study drug), and 164 (90.1%) completed the observational period to Week 48. Baseline demographics were similar across groups (Table A). At Week 24, all DZP groups showed numerically greater improvements in immunological and clinical outcomes vs PBO (Table B). Following study drug withdrawal, immunologic parameters generally worsened and returned towards baseline. Whereas SLEDAI and PGA stabilized across treatment groups after study drug withdrawal (Table B), BICLA and SRI-4 response rates declined, mostly due to interventions with escape medicines during this period, which automatically led to non-responder status. As none of the prespecified dose-response models fit the Week 24 BICLA responder rates with statistical significance (p< 0.05), the primary objective was not met. Rates of treatment-emergent adverse events (TEAEs) and serious TEAEs were generally balanced across treatment groups (Table C). Four thromboembolic TEAEs were observed during the DB period: one in the 24 mg/kg DZP group and three in PBO.
Conclusion: DZP-treated patients showed consistent improvements in disease activity across all doses. Upon study drug withdrawal, immunologic parameters returned to baseline levels, whilst clinical outcomes such as SLEDAI and PGA stabilized. None of the prespecified dose-response models could be selected; thus, the primary endpoint was not met. DZP was generally well-tolerated. The potential clinical benefit of DZP warrants investigation in a larger study.
To cite this abstract in AMA style:
Furie R, Bruce I, Dörner T, Leon M, Leszczyński P, Urowitz M, Haier B, Brittain C, Liu J, Barbey C, Stach C. Efficacy and Safety of Dapirolizumab Pegol in Patients with Moderately to Severely Active Systemic Lupus Erythematosus: A Randomized, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-dapirolizumab-pegol-in-patients-with-moderately-to-severely-active-systemic-lupus-erythematosus-a-randomized-placebo-controlled-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-dapirolizumab-pegol-in-patients-with-moderately-to-severely-active-systemic-lupus-erythematosus-a-randomized-placebo-controlled-study/