Session Information
Date: Monday, November 14, 2022
Title: Abstracts: SLE – Treatment
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Sphingosine-1-phosphate (S1P) regulates lymphocyte egress from lymphoid organs. In an SLE proof-of-concept study, cenerimod—a potent, selective S1P receptor modulator—reduced lymphocyte count and disease activity (measured by a SLEDAI-2K modified to exclude leukopenia [mSLEDAI-2K]) vs placebo (PBO) (Hermann. Lupus Sci Med 2019;6:e000354). The current phase 2 study (CARE) evaluated efficacy and safety of 4 cenerimod doses in moderate to severe SLE (NCT03742037).
Methods: Patients with SLE, mSLEDAI-2K ≥6 and current or past ANA or anti-dsDNA were randomized to daily oral cenerimod (0.5, 1, 2 or 4 mg) or PBO. Background SLE medication had to be stable for ≥30 days pre-randomization (corticosteroids ≥15 days).
Study duration was 18 months (M), two 6M treatment periods and a 6M follow-up. After the first 6M, patients on cenerimod 4 mg were rerandomized to cenerimod 2 mg or PBO to assess reversibility of lymphopenia and potential withdrawal effects.
The primary endpoint was change from baseline (BL) to M6 in mSLEDAI-2K. Secondary endpoints were SLE Responder Index SRI-4 and BILAG-2004 improvement. Safety endpoints included adverse events (AEs) and AEs of special interest (AESI). The Hochberg procedure was used to compare endpoints within each endpoint family and hierarchically within each dose across endpoints to preserve a 5% 2-sided Type I family-wise error rate.
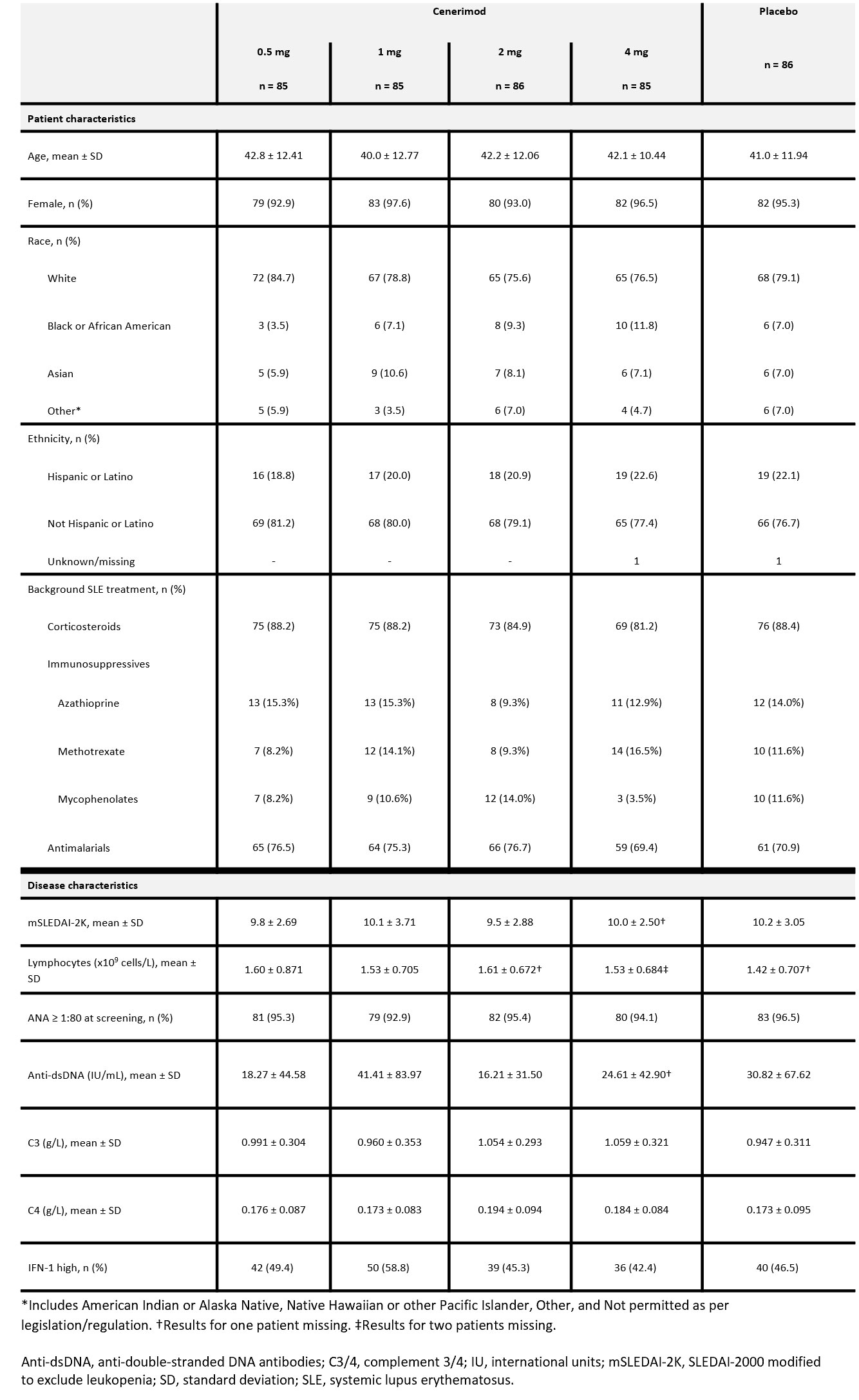
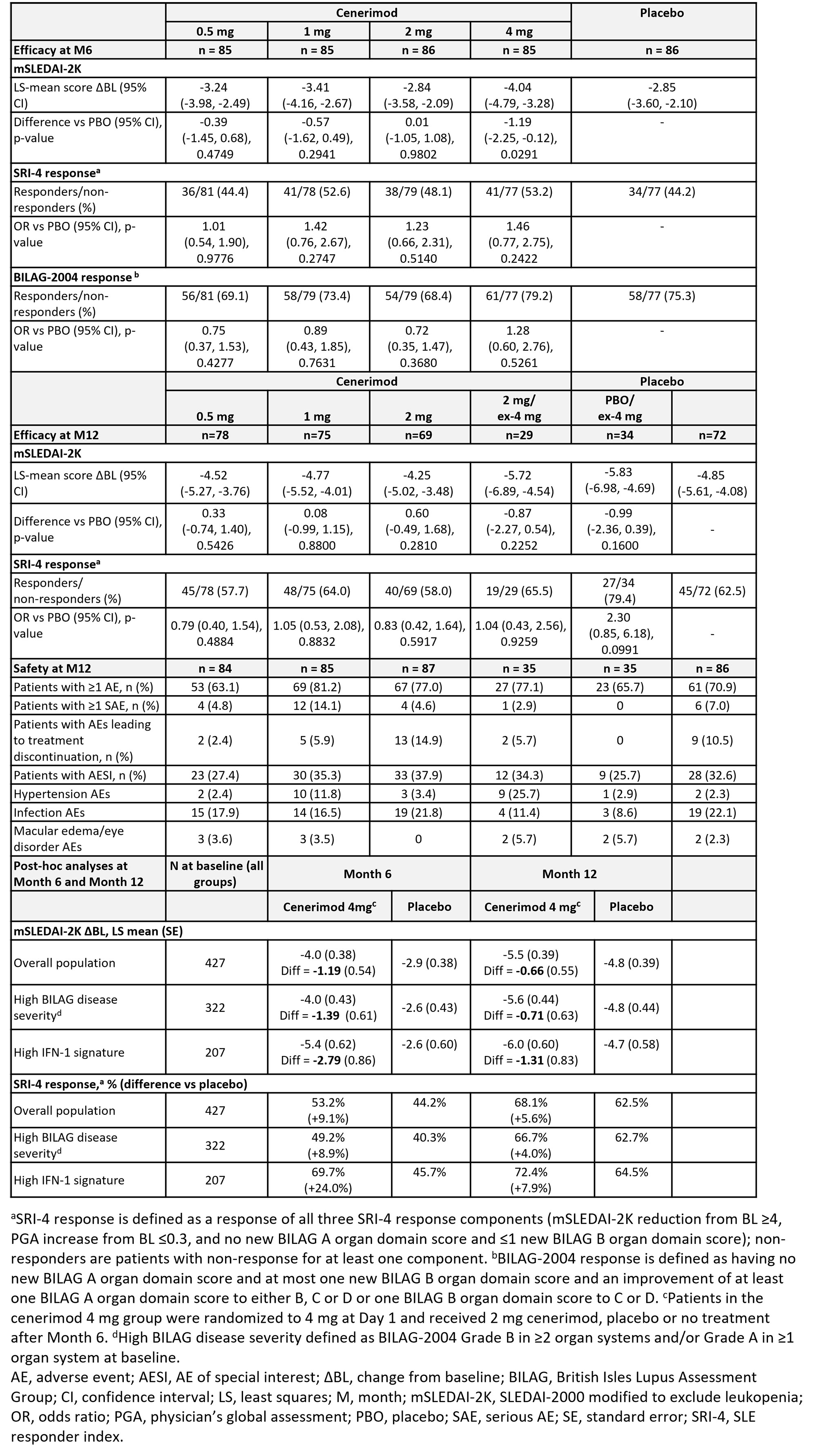
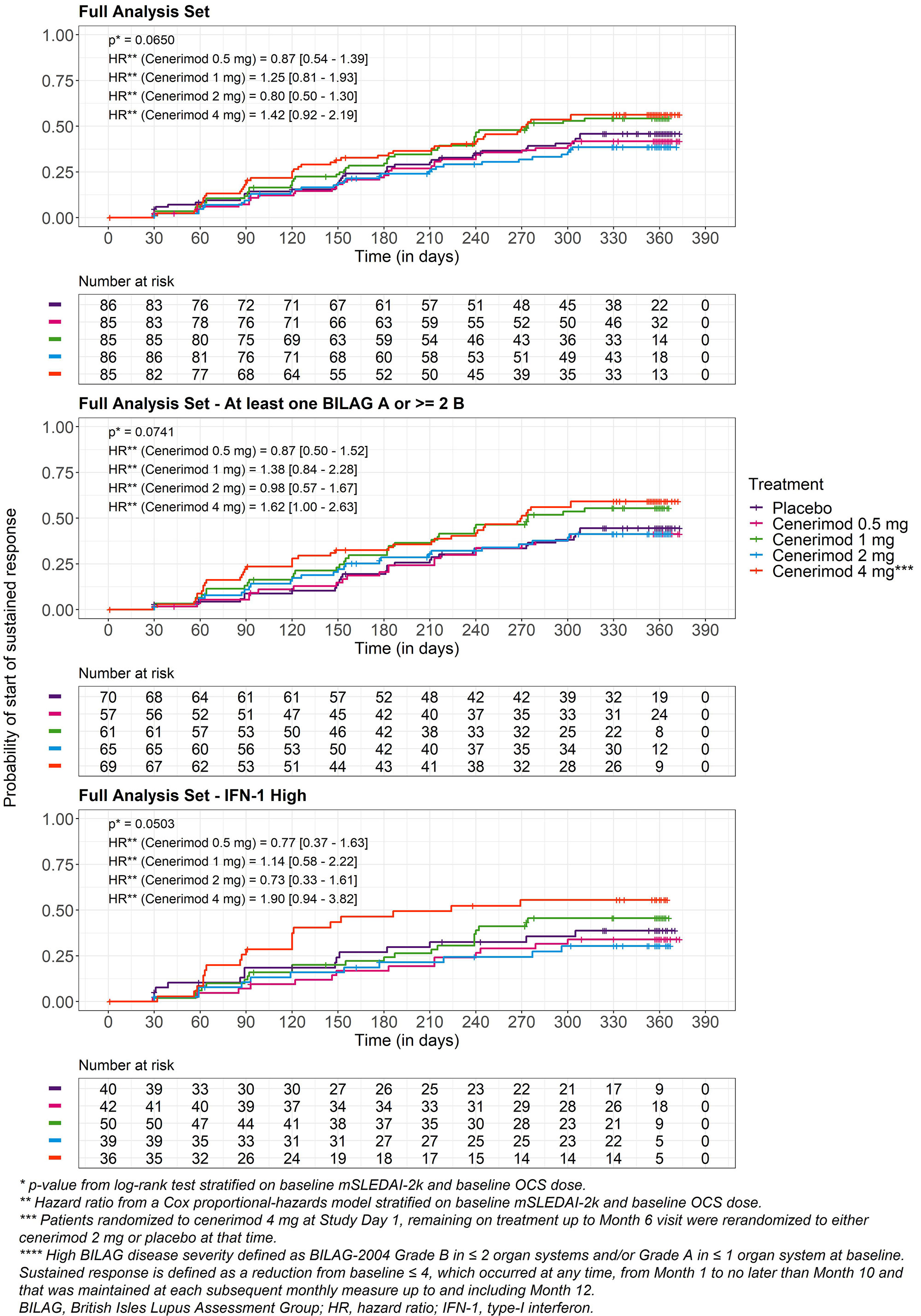
Results: Of 427 randomized patients, 339 completed 12M of treatment. BL characteristics were balanced across groups (Table 1). The study did not meet its primary endpoint after type I error control. In an exploratory analysis the reduction in mSLEDAI-2K from BL to M6 at cenerimod 4 mg vs PBO was statistically significant without accounting for type I error control; least squares [LS] mean difference (95% CI) -1.19(-2.25, -0.12), nominal P=0.0291 (Table 2). This effect was greater in patients with greater disease severity (BILAG-2004 Grade B in ≥2 organ systems and/or Grade A in ≥1 organ system at BL) (LS mean difference [95%CI] -1.39[-2.59, -0.19]) and high IFN-1 gene expression signature status (LS mean difference [95%CI] -2.79[-4.50, -1.08]) vs the overall population. At M6 and M12, the proportion of SRI-4 responders was higher in patients randomized to cenerimod 4 mg in the first 6M. This difference was also greater in high IFN-1 patients (difference +24% at M6 and +8% at M12). Sustained mSLEDAI-2K response started earlier in patients on cenerimod 4 mg vs PBO; this difference was greater in the IFN-1 high subgroup vs the overall population (Figure). Lymphocyte count decreased in all cenerimod groups; greater decreases were seen with the 2 and 4 mg doses and recovered by M12 in those rerandomized to PBO.
AEs were similar across treatment groups (Table 2). The % of reported hypertension AEs was higher in the cenerimod 1 and 4 mg groups vs PBO; however monthly objective measurements showed no increases in mean systolic or diastolic blood pressure. AESI were mild and transient; 1 death due to acute coronary syndrome occurred (1 mg group).
Conclusion: Cenerimod in patients with SLE was generally well tolerated. Exploratory analysis suggests that cenerimod 4 mg might improve disease and warrants further study, especially in IFN-1 high patients and greater disease severity.
To cite this abstract in AMA style:
Askanase A, Berkani O, Cahuzac c, Cornelisse p, D'Cruz D, Kalunian K, Merrill J, Pozzobon M, Navarra S. Efficacy and Safety of Cenerimod in Patients with Moderate to Severe Systemic Lupus Erythematosus (SLE): A Multicenter, Randomized, Parallel-Group, Double-Blind, Placebo-Controlled, Dose-Finding Phase 2b Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-cenerimod-in-patients-with-moderate-to-severe-systemic-lupus-erythematosus-sle-a-multicenter-randomized-parallel-group-double-blind-placebo-controlled-dose-finding-phase/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-cenerimod-in-patients-with-moderate-to-severe-systemic-lupus-erythematosus-sle-a-multicenter-randomized-parallel-group-double-blind-placebo-controlled-dose-finding-phase/