Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Although various biologic agents (BAs) are in use for polyarticular juvenile idiopathic arthritis (pJIA), a combined meta-analytic summary comparing the efficacy and safety among them in pJIA is lacking. An often applied trial design in pJIA, called withdrawal trials, consists of an open-label run-in phase where all patients receive active drug. After this phase, only those who respond to treatment enter the double-blind phase and are randomized to continue on drug or on placebo. Our objective was to compare the efficacy and safety of BAs in pJIA using all currently available randomized withdrawal trials (wRCTs) [1].
Methods:
A systematic search of MEDLINE, EMBASE, CENTRAL and clinicaltrials.gov was performed. Eligible wRCTs included patients with pJIA where BAs, at any dose, were compared with another BA or placebo. Efficacy was evaluated with disease flare (as defined by the authors) and ACRPedi30, 50, and 70. Safety was evaluated by examining patients with adverse events (AEs) and serious AEs (SAEs). Two reviewers extracted data, and a third confirmed. A network meta-analysis was used to compare BAs, based on a mixed-effects logistic regression model (modeled in SAS) [2]. This approach combined statistical inference from both direct and indirect comparisons of the treatment effects between BAs. Results are reported as odds ratios with 95% confidence intervals (OR [95%CI]).
Results:
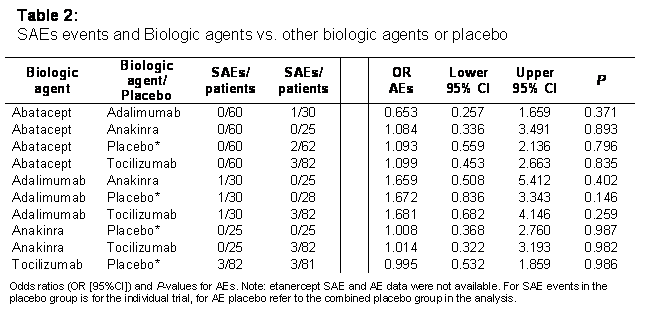
Of 490 references identified, 23 were reviewed in detail and 5 wRCTs were included: abatacept, adalimumab, anakinra, etanercept and tocilizumab, one trial each, all vs. placebo. Nearly all placebo comparisons showed statistically significant efficacy (P < 0.05) for flare (Table 1) and for ACRPedi 30, 50 and 70; the anakinra trial did not report ACRpedi measures and etanercept was not different for ACRpedi70 (2.30 [0.73;7.29]; P = 0.16). There were no differences (P > 0.05) among BAs for efficacy. SAEs occurred very infrequently (0-8%) and an analysis was not possibly (Table 2). As noted in Table 2 there were no differences for AEs when compared to placebo or among BAs.
Conclusion:
Overall BAs were effective and safe when compared placebo. There were no differences among BAs for either efficacy or safety. Based on these data, other considerations such as price and availability should be used to decide among BAs when treating pJIA patients as no personalized biomarkers etc. are available at present. Long term safety will be better examined in the biologic registries currently accruing data in JIA.
References:
[1] Amarilyo G, et al. PROSPERO. 2013;CRD42013004736
[2] Singh JA, et al. CMAJ. 2009;181(11):787-96
Disclosure:
G. Amarilyo,
None;
S. Tarp,
None;
I. Foeldvari,
Abbott, Chugai,
5,
Pizer,
8;
N. Cohen,
None;
T. D. Pope,
None;
J. M. P. Woo,
None;
R. Christensen,
Abbott, Axellus A/S, Bristol-Myers Squibb, Cambridge Weight Plan, Norpharma, Pfizer, and Roche,
5,
Axellus A/S, Cambridge Weight Plan, Mundipharma, and Roche,
2,
Abbott, Axellus, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Cambridge Weight Plan, Ipsen, Laboratoires Expanscience, MSD, Mundipharma, Norpharma, Pfizer, Roche, and Wyeth,
8;
D. Furst,
AbbVie, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
2,
AbbVie, Actelion, Amgen, BMS, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
5,
AbbVie, Actelion, UCB ,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-biologic-agents-in-patients-with-poly-articular-juvenile-idiopathic-arthritis-network-meta-analysis-of-randomized-controlled-withdrawal-trials/