Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Efficacy and safety of a new subcutaneous (SC) formulation (CT-P13 SC) were evaluated up to Week 30. The phase I/III randomized controlled trial in patients with active rheumatoid arthritis (RA) study demonstrated non-inferiority of efficacy (mean change [decrease] from baseline in DAS28 [CRP] at Week 22) for CT-P13 SC 120 mg versus CT-P13 IV 3 mg/kg and showed similar safety profile between 2 arms [1]. This is to investigate the efficacy and safety of CT-P13 SC when used over 1-year and after switching from CT-P13 IV in patients with active RA.
Methods: In this randomized, controlled, double-blinded, phase I/III study, patients who received full doses of CT-P13 IV 3 mg/kg at Weeks 0 and 2 were randomly assigned to receive either CT-P13 SC 120 mg via pre-filled syringe biweekly or CT-P13 IV 3 mg/kg every 8 weeks from Week 6 to Week 28. From Week 30, all patients received CT-P13 SC 120 mg via pre-filled syringe biweekly up to Week 54. Efficacy and safety were evaluated for 54 Weeks.
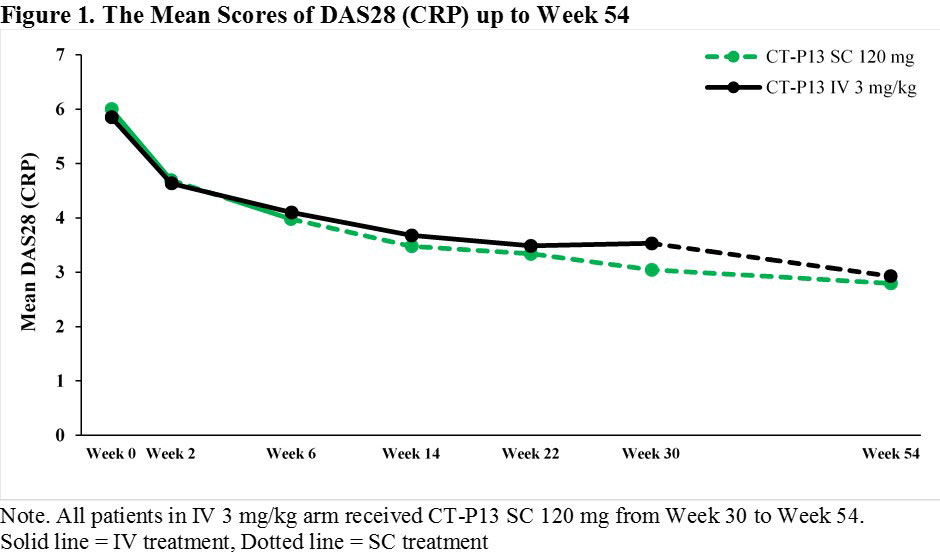
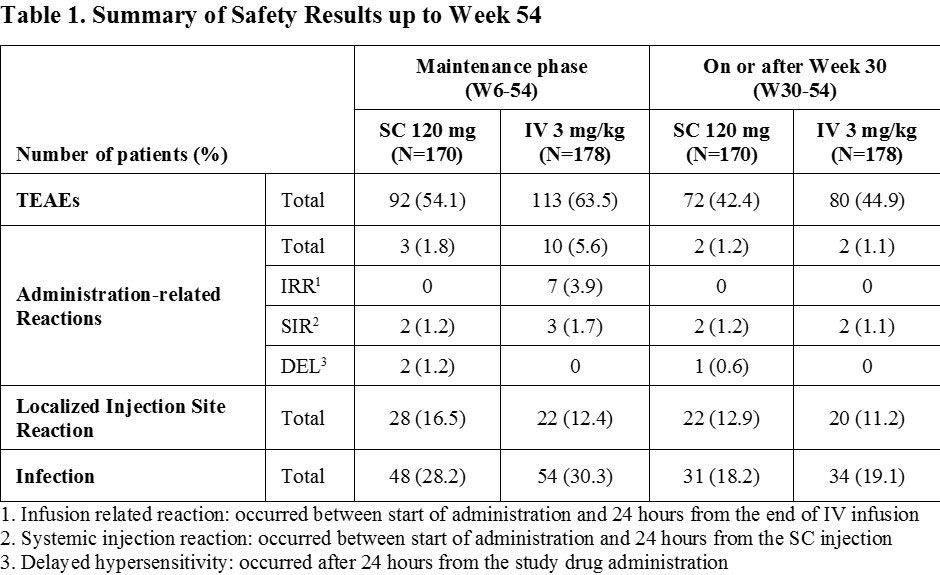
Results: A total of 362 patients were enrolled, of whom 348 patients were randomly assigned at Week 6 into 2 arms in a 1:1 ratio (169 and 179 patients in SC 120 mg and IV 3 mg/kg arms, respectively). The mean DAS28 (CRP) and ACR response rates were similar between 2 arms up to Week 22 with a slightly greater response in SC 120 mg arm at Week 30. After switching from CT-P13 IV 3 mg/kg to CT-P13 SC 120 mg at Week 30 in IV 3 mg/kg arm, the mean DAS28 (CRP) was similar between 2 arms (Figure 1) whereas ACR response rates were slightly higher in SC 120 mg arm compared to IV 3 mg/kg arm at Week 54 (Figure 2). The safety profiles which occurred on or after Weeks 6 and 30 in SC 120 mg arm were generally comparable to IV 3 mg/kg arm. The majority of the localized injection site reactions were grade 1 or 2 in intensity (Table 1).
Conclusion: The effectiveness and tolerability were confirmed over the 1-year treatment of CT-P13 SC 120 mg. The results after switching from CT-P13 IV 3 mg/kg to CT-P13 SC 120 mg at Week 30 were comparable to that of maintaining CT-P13 SC 120 mg up to Week 54. These results show that the novel SC formulation of CT-P13 via pre-filled syringe could provide a favorable benefit to patients with an alternative convenient way of administration.
References:
[1] R. Westhovens, et al., Ann Rheum Dis, volume 78, supplement 2, year 2019, page A1158
To cite this abstract in AMA style:
Westhovens R, Wiland P, Zawadzki M, Ivanova D, Berrocal A, Chalouhi E, Balázs �, Shevchuk S, Eliseeva L, Stanislavchuk M, Yatsyshyn R, Lee S, Kim S, Han N, Jung Y, Yoo D. Efficacy and Safety of a Novel Subcutaneous Formulation of CT-P13 over the 1-year Treatment Period and After Switching from Intravenous CT-P13 in Patients with Active Rheumatoid Arthritis: Results from Part 2 of Phase I/III Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-a-novel-subcutaneous-formulation-of-ct-p13-over-the-1-year-treatment-period-and-after-switching-from-intravenous-ct-p13-in-patients-with-active-rheumatoid-arthritis-results-fro/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-a-novel-subcutaneous-formulation-of-ct-p13-over-the-1-year-treatment-period-and-after-switching-from-intravenous-ct-p13-in-patients-with-active-rheumatoid-arthritis-results-fro/