Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
NSAIDs are one of the most used drug classes in the world.1 However, its use may be associated with potentially limiting adverse events (AEs), especially gastrointestinal toxicity.2 Concomitant use of proton-pump inhibitors, including fixed-dose combination regimens, have emerged as an alternative to minimize gastrointestinal AEs.3 This study investigated the safety and efficacy of fixed-dose combination of naproxen/esomeprazole and nimesulide/pantoprazole to determine if both regimens are equally suited to relieve pain in patients with osteoarticular disease and dyspeptic symptoms.
Methods:
This was a multi-center, randomized, double-blind, active-controlled, parallel-group, non-inferiority phase 3 study. Patients were assigned to receive either nimesulide/pantoprazole (100mg/20mg) twice daily or naproxen/esomeprazole (500mg/20mg) twice daily for 14 days. The primary endpoint was the mean change in modified Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)4 pain subscale. Satefy assessment was performed by the mean visual analog scale (VAS) of dyspeptic symptoms. This study is registered at ClinicalTrials.gov: NCT01670552.
Results:
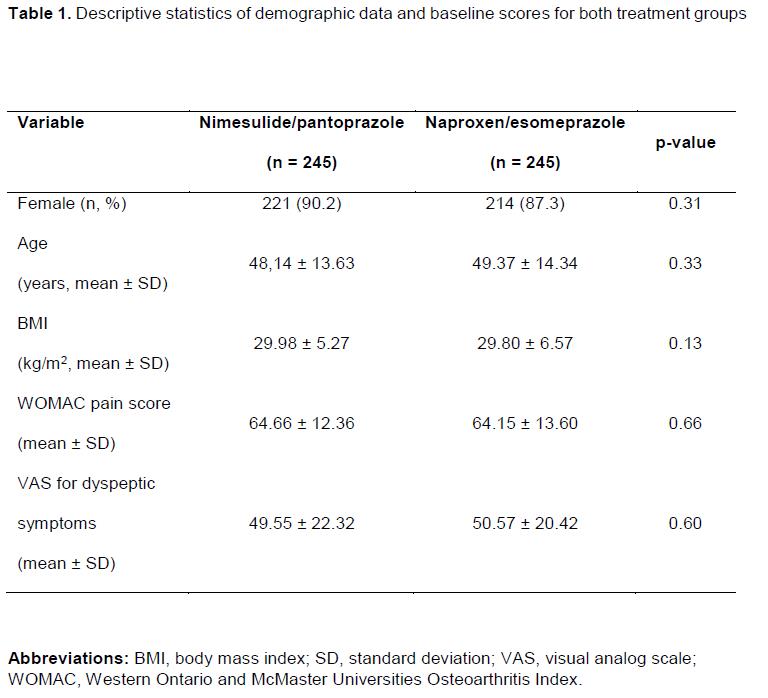
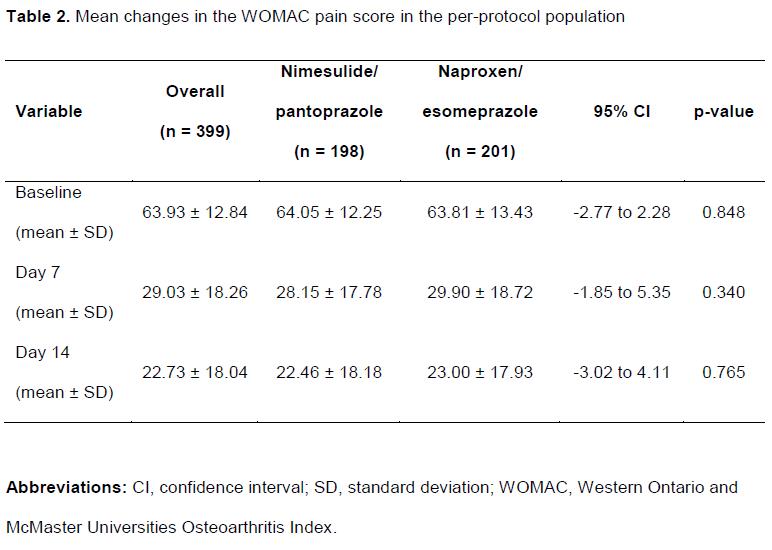
A total of 490 patients were enrolled and randomized (399 completed). The baseline characteristics are presented in Table 1. Mean changes in the modified WOMAC are presented in Table 2. The difference in mean change in the modified WOMAC after 7 days of treatment between the 2 groups was 2.33 mm (95% CI, -1.22 to 5.89 mm). After 14 days of therapy, the difference was 0.45 mm (95% CI, -3.29 to 4.19 mm). Overall frequencies of AEs were similar in the 2 groups.
Conclusion:
The present study demonstrated non-inferiority of a 14-day regimen with a fixed-dose combination of nimesulide/pantoprazole compared to naproxen/esomeprazole for the treatment of osteoarticular pain. The results of the study show that the gastrointestinal adverse effects related to NSAID use may be reduced by the use of a fixed-dose combination of nimesulide/pantoprazole.
References:
1. Fine M. Quantifying the impact of NSAID-associated adverse events. American Journal of Managed Care. 2013; 19.
2. Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. American Journal of Medicine. 1998.
3. Scheiman JM. The use of proton pump inhibitors in treating and preventing NSAID-induced mucosal damage. Arthritis Res Ther. 2013;15(Suppl 3):S5.
4. Bellamy N. WOMAC osteoarthritis index user guide. Version V. Brisbane, Aust. 2002.
To cite this abstract in AMA style:
de Almeida Macêdo E, Pott Junior H, Scheinberg M, Ecclissato C, Bleuel Amazonas R. Efficacy and Safety of a Fixed-Dose Combination of Nimesulide/Pantoprazole Compared with Naproxen/Esomeprazole for Pain Relief in Patients with Osteoarticular Diseases and Dyspeptic Symptoms [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-a-fixed-dose-combination-of-nimesulide-pantoprazole-compared-with-naproxen-esomeprazole-for-pain-relief-in-patients-with-osteoarticular-diseases-and-dyspeptic-symptoms/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-a-fixed-dose-combination-of-nimesulide-pantoprazole-compared-with-naproxen-esomeprazole-for-pain-relief-in-patients-with-osteoarticular-diseases-and-dyspeptic-symptoms/