Session Information
Date: Tuesday, November 10, 2015
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment VI: Novel Therapies
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Arsenic Trioxide (ATO) is approved for the treatment
of acute promyelocytic leukemia, increasing oxidative stress with selective
apoptosis of leukemia cells. In MLR/lpr model of SLE, ATO demonstrated a
dramatic improvement. Given the promising results of experimental models, we
conducted this study in patients with SLE
Methods: The primary objective of this phase I/IIa proof-of-concept study was
to evaluate the safety and tolerability of IV ATO in patients with SLE. Efficacy
and pharmacokinetics were also evaluated. Patients with mild-to-moderate SLE,
defining as SELENA-SLEDAI score of ≥ 4 at baseline and corticosteroid
dosage > 10 mg/day with active disease despite standard-of-care treatment,
including hydroxychloroquine (HCQ) were enrolled. Concomitant therapy with
methotrexate (MTX), mycophenolate (MMF), and azathioprine (AZA) were allowed at
constant doses during the study. Patients with severe active renal or CNS
disease were excluded. Patients received 10 IV infusions of ATO from day1 to
day 4 than at day 8, 11, 14, 17, 21 and 25, with escalading dose from 0.10
mg/kg to 0.20 mg/kg, and follow-up for 20 weeks. The primary outcome measure
was safety assessment with recording of adverse events (AEs) according to NCI Common
Terminology Criteria for Adverse Events. Major secondary endpoint included disease
activity measures, assessed by SLE Responder Index (SLEDAI, Physician’s Global
Assessment (PGA) and BILAG : combination of SLEDAI response ≥ 4 points,
no BILAG A or 2 x B flares and no PGA score worsening, corticosteroid sparing.
Results: 11 patients (all on HCQ) are included to date in this
ongoing study, and were evaluated for safety and toxicity. Five among 11 received
MTX, 1 AZA, 1 thalidomide and 2 MMF. Dose of ATO was 0.10mg/kg/day in 4
patients, 0.15mg/kg/day in 4 and 0.2 mg/kg/day in 3. Two severe AEs occurred in
2 patients (0.15 and 0.2 mg/kg, receiving also MMF) with transient (<5 days)
asymptomatic grade 3 neutropenia (at day 15). ATO was discontinued in these patients
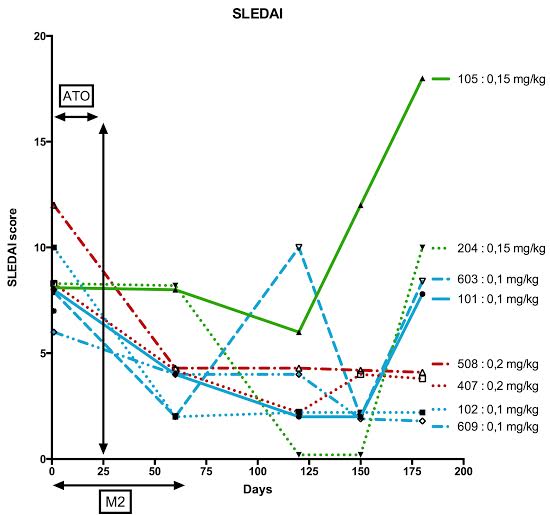
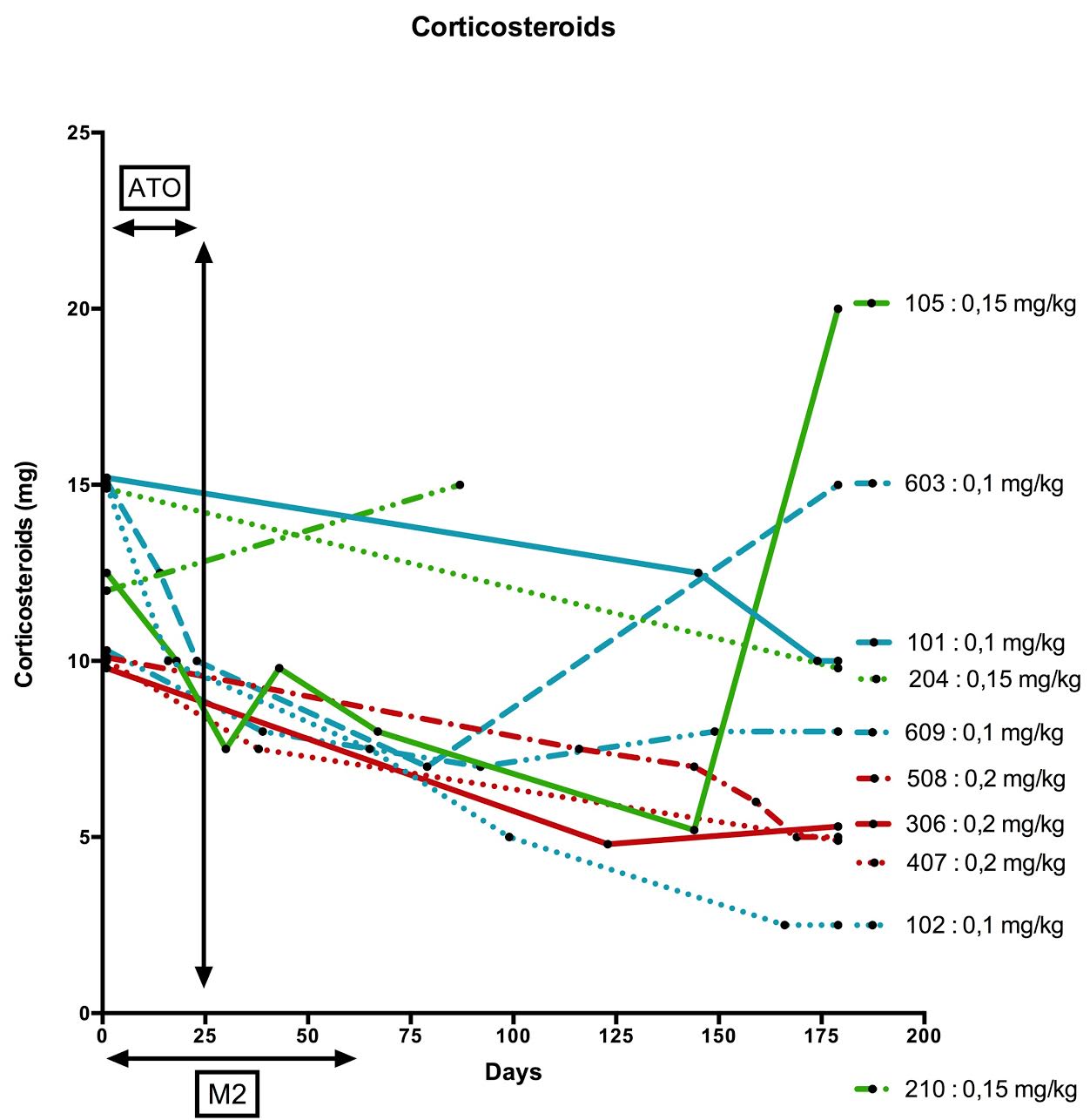
without MMF interruption. Mean SLEDAI at baseline was 8 and decreased to 4 at
Month 2 and 3 at Month 4, with steroid sparing (Figures).
Six patients among 8 had SRI
response, generally without improvement of anti-dsDNA antibodies and C3/C4.
Conclusion: In this proof-of-concept phase 1/IIa study, 4 weeks IV
ATO treatment with 20 weeks follow-up, demonstrated an acceptable safety and
tolerability profile (except in patients treated by MMF) with a clinical
efficacy, supporting further evaluation in larger clinical trials.
.
To cite this abstract in AMA style:
Hamidou M, Gaborit B, Hachulla E, Amoura Z, Ebbo M, Chatelus E, Sibilia J, Viallard JF, Graveleau J, Saint Jean M, Gardes S, Foucher Y, Volteau C, Poupon J, Neel A, Rieger F. Efficacy and Safety of 4 Weeks Administration of Arsenic Trioxide in Moderate Active Systemic Lupus Erythematosus. a Phase I/II Proof-of-Concept Sequential Dose Escalation Multicenter Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-4-weeks-administration-of-arsenic-trioxide-in-moderate-active-systemic-lupus-erythematosus-a-phase-iii-proof-of-concept-sequential-dose-escalation-multicenter-study/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-4-weeks-administration-of-arsenic-trioxide-in-moderate-active-systemic-lupus-erythematosus-a-phase-iii-proof-of-concept-sequential-dose-escalation-multicenter-study/