Background/Purpose: Odanacatib (ODN) is a potent, orally-active cathepsin K inhibitor being developed for the treatment of postmenopausal osteoporosis. This study evaluated the effects of ODN 50mg once weekly (OW) on BMD and biochemical markers of bone turnover in patients previously treated with alendronate (ALN) (dosed daily or weekly) for ≥3years, as well as the safety and tolerability of ODN.
Methods: This was a randomized, double-blind, placebo-controlled, 24-month study. The primary endpoint was % change in femoral neck (FN) BMD from baseline at Month 24. 243 postmenopausal women ≥60 years of age with low BMD T-score (T-score range ≤–2.5 but >-3.5) at the total hip, FN or trochanter but no history of hip fracture and who had been treated with ALN for ≥3years were randomized in a 1:1 ratio to receive ODN 50mg OW or placebo OW for 24 months. All patients received vitamin D3 5600 IU/wk and calcium supplementation (to 1200 mg/day). BMD was assessed by DXA at baseline, 6, 12 and 24 months. Biochemical markers of bone resorption (s-CTx, u-NTx) and bone formation (s-BSAP and s-P1NP) were measured at baseline and 3, 6, 12, 18 and 24 months. This study was not designed and did not have the power to evaluate the effect of ODN on fractures.
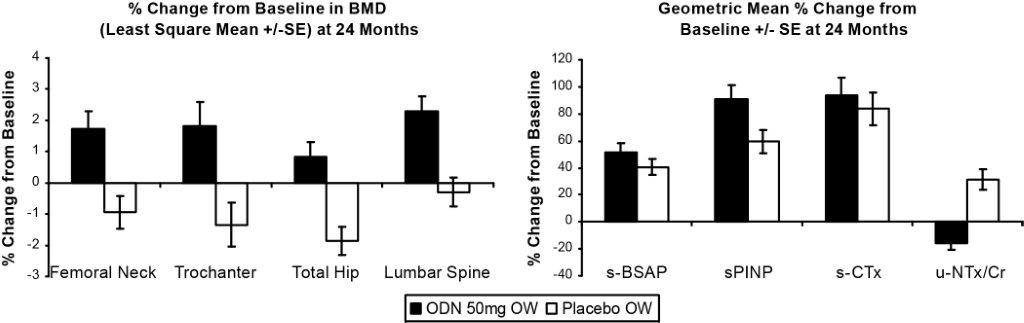
Results: In the placebo group, FN and trochanter BMD were not significantly different from baseline levels for the first 12 months, but declined significantly from baseline by Month 24 (-0.94% and -1.35%, respectively). BMD at the total hip declined in a linear manner from baseline to month 24 (-1.87% at 24 months). BMD at the lumbar spine (LS) was not significantly different from baseline for the entire 24 months of the study. BMD changes from baseline at 24 months in the ODN group were significant vs placebo at all 3 hip sites and the LS. The changes in BMD for the FN, trochanter, total hip and LS from baseline were 1.73%, 1.83%, 0.83% and 2.28%, respectively. ODN 50mg OW significantly decreased the biomarker of bone resorption, u-NTx/Cr, and significantly increased biomarkers of bone formation, s-P1NP and s-BSAP, compared to placebo. The increase observed for the bone resorption marker s-CTx with ODN treatment was unexpected. AEs were comparable between the 2 treatment arms. The overall safety profile appeared similar between ODN 50mg OW and placebo.
Conclusion: In this study osteoporotic women treated with ODN following ALN treatment show incremental gains in BMD. Biomarker results suggest that ODN decreases bone resorption while preserving bone formation.
ODN effects on BMD
ODN effects on biomarkers
Disclosure:
R. Chapurlat,
Merck Pharmaceuticals,
2,
Merck Pharmaceuticals,
5;
S. Bonnick,
Merck Pharmaceuticals,
5;
T. De Villiers,
None;
A. Odio,
Merck Pharmaceuticals,
2;
S. Palacios,
None;
B. Scott,
Merck Pharmaceuticals,
3,
Merck Pharmaceuticals,
1;
C. Le Bailly De Tilleghem,
Merck Pharmaceuticals,
3,
Merck Human Health,
1;
C. DaSilva,
Merck Pharmaceuticals,
3,
Merck Pharmaceuticals,
1;
A. Leung,
Merck Pharmaceuticals,
1,
Merck Pharmaceuticals,
3;
D. Gurner,
Merck Pharmaceuticals,
1,
Merck Pharmaceuticals,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-odanacatib-on-bmd-and-overall-safety-in-the-treatment-of-osteoporosis-in-postmenopausal-women-previously-treated-with-alendronate/