Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
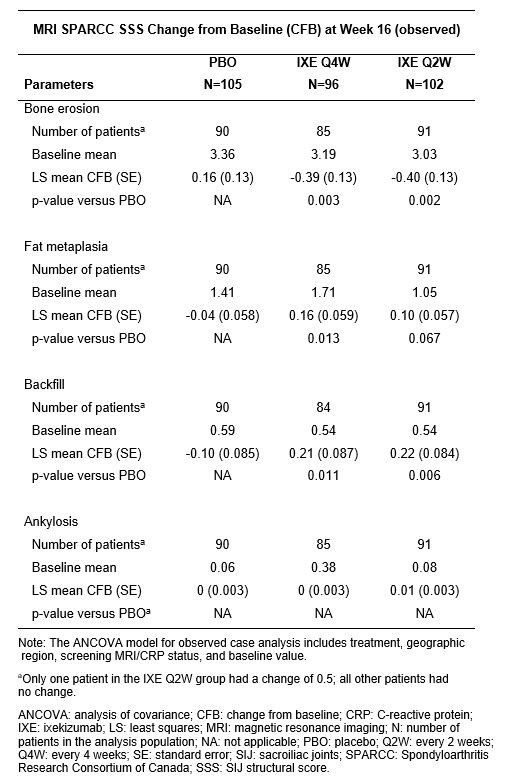
Background/Purpose: To evaluate the effect of treatment with ixekizumab versus placebo (PBO) on structural lesions in the sacroiliac joints (SIJ) at 16 weeks in patients with non-radiographic axial spondyloarthritis (nr-axSpA).
Methods: Patients with active nr-axSpA who were biologic-naïve (COAST-X, NCT02757352) were randomized 1:1:1 to receive double-blinded ixekizumab 80 mg every 4 (Q4W) or 2 weeks (Q2W) with an 80-mg or 160-mg starting dose at week 0 or PBO. SIJ magnetic resonance imaging (MRI) was assessed by Spondyloarthritis Research Consortium of Canada (SPARCC) SIJ structural score (SSS). Treatment comparisons used analysis of covariance based on observed cases.
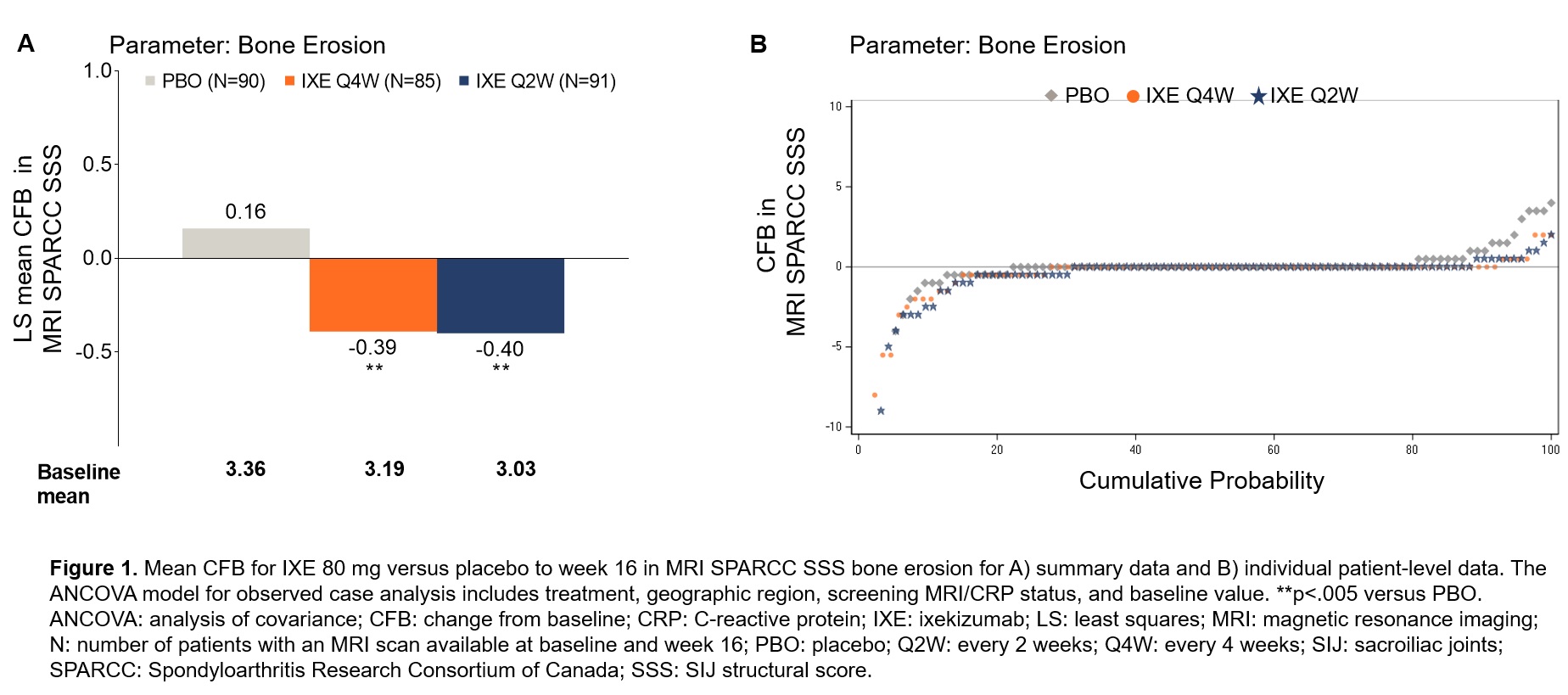
Results: Of 303 randomized patients, 266 patients (Q4W: n=85, Q2W: n=91, PBO: n=90) with an MRI scan available at baseline and week 16 were included in this analysis. Mean SPARCC SSS for PBO and ixekizumab dose groups were similar at baseline (Figure 1 and Table). Significant reduction in SPARCC SSS for erosion was observed in both ixekizumab dose groups versus PBO (Fig. 1A). Increased erosion scores occurred for fewer patients in both ixekizumab dose groups versus PBO (Fig. 1B). Decreased erosion scores occurred for more patients in both ixekizumab dose groups versus PBO (Fig. 1B). Mean change from baseline in SPARCC SSS was significantly greater for ixekizumab for fat metaplasia (Q4W) and backfill (Q4W and Q2W) versus PBO (Table). Changes in ankylosis were generally not observed (Table).
Conclusion: Treatment with ixekizumab versus PBO led to significant reductions in erosions and significant increases in fat metaplasia and backfill in the SIJ in patients with nr-axSpA assessed at 16 weeks of treatment.
To cite this abstract in AMA style:
Maksymowych W, Baraliakos X, Lambert R, Landewé R, Sandoval Calderon D, Carlier H, Lisse J, Li X, Hojnik M, Ostergaard M. Effects of Ixekizumab Treatment on Structural Changes in the Sacroiliac Joints Based on MRI Assessments at 16 Weeks in Patients with Non-Radiographic Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effects-of-ixekizumab-treatment-on-structural-changes-in-the-sacroiliac-joints-based-on-mri-assessments-at-16-weeks-in-patients-with-non-radiographic-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-ixekizumab-treatment-on-structural-changes-in-the-sacroiliac-joints-based-on-mri-assessments-at-16-weeks-in-patients-with-non-radiographic-axial-spondyloarthritis/