Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with ankylosing spondylitis (AS), IV administration of the anti-TNFa antibody golimumab (GLM-IV) resulted in improvements in composite measures of various aspects of the disease (eg, ASAS percent response, BASDAI, and BASFI) that were greater than placebo (PBO) at week 16 or earlier in the GO-ALIVE study.1 The improvements were maintained for up to 1 year of treatment.2 Here we examine treatment effects on health-related quality of life (HRQoL).
Methods: Adult patients with definite AS (per modified NY criteria), BASDAI ≥4, total back pain VAS ≥4, CRP ≥0.3 mg/dL, and inadequate response to NSAIDs were randomized to GLM-IV 2mg/kg at weeks 0 and 4 then every 8 weeks, or to PBO at weeks 0 and 4 and GLM-IV at weeks 16 and 20, then every 8 weeks. Stable doses of methotrexate (≤ 25 mg/week), sulfasalazine, hydroxychloroquine, NSAIDs, other analgesics, and low dose oral corticosteroids were permitted for patients who were receiving these medications at baseline. Measures of HRQoL included the Ankylosing Spondylitis Quality of Life questionnaire (ASQoL), Short Form-36 physical and mental component summary scores (SF-36 PCS/MCS), Medical Outcomes Study Sleep Scale (MOS-SS), and EuroQoL visual analog scale (EQ VAS), each measured at weeks 16, 28, and 52. P values provided are nominal, not adjusted for multiplicity.
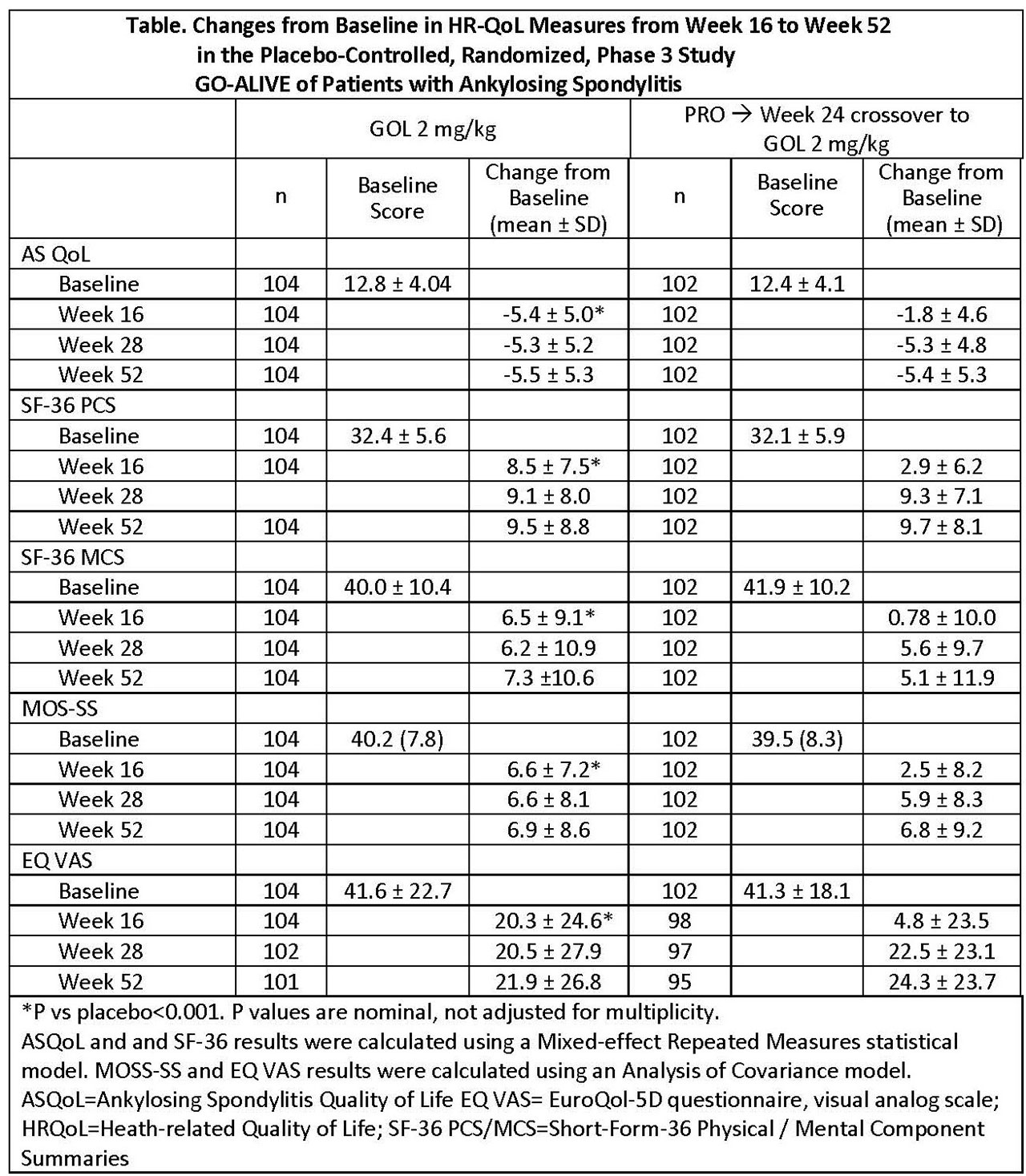
Results: At week 16, patients with AS receiving GLM-IV had greater improvements from baseline in HRQoL than those receiving PBO in each measure, respectively (ASQoL, -5.4 vs -1.8; SF-36 PCS, 8.5 vs 2.9; SF-36 MCS, 6.5 vs 0.78; MOS-SS, 6.6 vs 2.5; and EQ VAS, 20.3 vs 4.8; all p< 0.001), see Table. Changes from baseline were maintained through week 52 in patients randomized to GLM-IV. Patients switched from PBO to GLM IV at week 16 demonstrated improvement from baseline by week 28, which was maintained through week 52 and was similar to that of patients who received GLM IV at baseline (Table).
Conclusion: Improvements in HRQoL among patients with active AS treated with GLM-IV were greater than PBO at week 16 and were maintained through week 52. Patients switching from PBO to GLM-IV at week 16 experienced improvements in HRQoL by week 28 and maintained the improvement through week 52 at levels similar to those of the patients originally randomized to GLM-IV.
- Deodhar et al. J Rheum. 2018;45:341
- Reveille et al. J Rheum. 2019. DOI: 10.3899/jrheum.180718.
To cite this abstract in AMA style:
Reveille J, Deodhar A, Harrison D, Hsia E, Chan E, Kafka S, Lo K, Kim L, Han C. Effects of Intravenous Golimumab, an Anti-TNFα Monoclonal Antibody, on Health-Related Quality of Life in Patients with Ankylosing Spondylitis: 1-Year Results of a Phase III Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effects-of-intravenous-golimumab-an-anti-tnf%ce%b1-monoclonal-antibody-on-health-related-quality-of-life-in-patients-with-ankylosing-spondylitis-1-year-results-of-a-phase-iii-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-intravenous-golimumab-an-anti-tnf%ce%b1-monoclonal-antibody-on-health-related-quality-of-life-in-patients-with-ankylosing-spondylitis-1-year-results-of-a-phase-iii-trial/