Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: One of the most feared complications of giant cell arteritis (GCA) is visual affection. Tocilizumab (TCZ) has demonstrated efficacy and safety in GCA. However, data on visual affection with TCZ are scarce and sometimes contradictory.
Our objectives were to evaluate the efficacy of TCZ in a)preventing the onset of new visual manifestations, and b)improving visual symptoms when present.
Methods: Multicenter observational study of 471 patients with GCA treated with TCZ of clinical practice. Patients were diagnosed with GCA according to a)ACR criteria, and/or b)temporal artery biopsy, and/or c)imaging techniques.
Patients were divided into 2 groups: a)with visual involvement at some time during the disease, and b)without visual involvement. Visual manifestations were classified as: a)transient visual loss (TVL) (amaurosis fugax), b)permanent visual loss (PVL) (more than 24 hours) (partial or complete; unilateral or bilateral), c)diplopia, and d)blurred vision. According to the duration between the onset of visual symptoms and the onset of TCZ we have considered a)1-10 days, b)11-30 days, and c)more than 30 days.
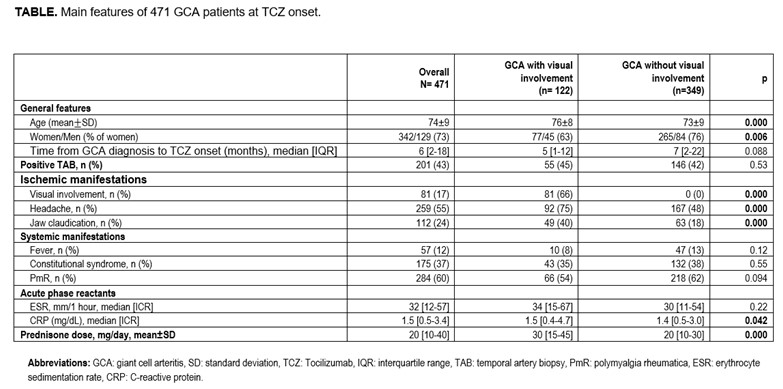
Results: We studied 471 (342 women/ 129 men; mean age: 74 ± 9 years). Visual manifestations at any time of the disease were observed in 122 (26%) patients. In 81 of them visual manifestations were present at TCZ onset, while the remaining 41 patients had had a complete recovery previously to TCZ. The main GCA features of patients with and without visual involvement are shown in TABLE. Patients with visual involvement were older, with other ischemic complications, and required higher doses of corticosteroids.
After starting TCZ, no patient developed new visual involvement. At TCZ onset, 81 patients had the following visual manifestations: PVL (n= 60; unilateral/bilateral: 48/12), TVL (n= 17; unilateral/bilateral: 11/6), diplopia (n=2) and blurred vision (n=2).
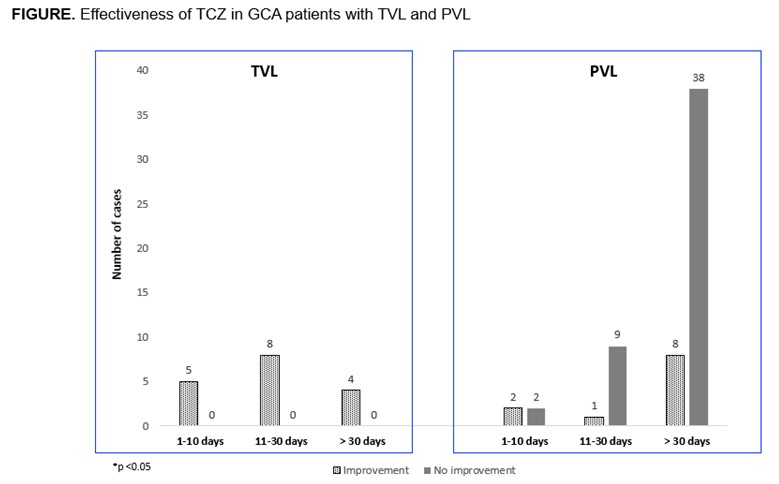
None of the patients with TVL had new episodes after initiation of TCZ, while 11 out of 60 patients with PVL experienced some improvement (FIGURE). The two patients with diplopia and one of the two patients with blurred vision improved.
Conclusion: TCZ seems to prevent the appearance of new ocular manifestations. When they are present, TCZ may improve totally TVL and partially PVL.
Background/Purpose: One of the most feared complications of giant cell arteritis (GCA) is visual affection. Tocilizumab (TCZ) has demonstrated efficacy and safety in GCA. However, data on visual affection with TCZ are scarce and sometimes contradictory.
Our objectives were to evaluate the efficacy of TCZ in a)preventing the onset of new visual manifestations, and b)improving visual symptoms when present.
Methods: Multicenter observational study of 471 patients with GCA treated with TCZ of clinical practice. Patients were diagnosed with GCA according to a)ACR criteria, and/or b)temporal artery biopsy, and/or c)imaging techniques.
Patients were divided into 2 groups: a)with visual involvement at some time during the disease, and b)without visual involvement. Visual manifestations were classified as: a)transient visual loss (TVL) (amaurosis fugax), b)permanent visual loss (PVL) (more than 24 hours) (partial or complete; unilateral or bilateral), c)diplopia, and d)blurred vision. According to the duration between the onset of visual symptoms and the onset of TCZ we have considered a)1-10 days, b)11-30 days, and c)more than 30 days.
Results: We studied 471 (342 women/ 129 men; mean age: 74 ± 9 years). Visual manifestations at any time of the disease were observed in 122 (26%) patients. In 81 of them visual manifestations were present at TCZ onset, while the remaining 41 patients had had a complete recovery previously to TCZ. The main GCA features of patients with and without visual involvement are shown in TABLE. Patients with visual involvement were older, with other ischemic complications, and required higher doses of corticosteroids.
After starting TCZ, no patient developed new visual involvement. At TCZ onset, 81 patients had the following visual manifestations: PVL (n= 60; unilateral/bilateral: 48/12), TVL (n= 17; unilateral/bilateral: 11/6), diplopia (n=2) and blurred vision (n=2).
None of the patients with TVL had new episodes after initiation of TCZ, while 11 out of 60 patients with PVL experienced some improvement (FIGURE). The two patients with diplopia and one of the two patients with blurred vision improved.
Conclusion: TCZ seems to prevent the appearance of new ocular manifestations. When they are present, TCZ may improve totally TVL and partially PVL.
To cite this abstract in AMA style:
Sánchez-Bilbao L, Loricera J, Valdivieso Achá J, Moriano C, Narvaez J, Aldasoro V, Maíz O, Melero R, Villa J, Vela P, Romero-Yuste S, Callejas J, De Miguel E, Galindez-Agirregoikoa E, Sivera F, Fernández-López J, Galisteo C, Ferraz-Amaro I, Nieto J, de Dios J, Sánchez J, Fernández E, de la Morena I, Moya P, Solans-Laqué R, Andreu J, Revenga M, Pinillos V, García-Valle A, Gallego A, Iñíguez C, Hidalgo C, Garrido-Puñal N, López-González R, Román-Ivorra J, Manrique Arija S, Collado P, Raya E, Navarro F, Mas A, Ordas C, Boquet M, Álvarez-Rivas N, Velloso-Feijoo M, Campos-Fernández C, Rúa-Figueroa , Conesa A, Salgado E, gonzalez-Gay M, Blanco R. Effectiveness of Tocilizumab in the Visual Involvement of Giant Cell Arteritis: Multicenter Study of 471 Patients of Clinical Practice [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-tocilizumab-in-the-visual-involvement-of-giant-cell-arteritis-multicenter-study-of-471-patients-of-clinical-practice/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-tocilizumab-in-the-visual-involvement-of-giant-cell-arteritis-multicenter-study-of-471-patients-of-clinical-practice/