Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Vaccine platforms, number of doses, and immunosuppressive drugs can influence the immunogenicity after SARS-CoV-2 vaccination in individuals with immune-mediated rheumatic diseases. Considering the heterogeneity of systemic vasculitides and their phenotypic variations according to geographic localization, there is still limited data about the immunogenic response in this population, with most studies focusing on homologous vaccine platforms. Furthermore, concerns regarding vaccine-induced vasculitides and potential disease relapse have affected vaccine hesitancy in these patients. This study evaluated the effectiveness of three doses (booster dose) of SARS-CoV-2 vaccines and the occurrence of disease relapse in systemic vasculitides.
Methods: We enrolled 99 patients with systemic vasculitides in a Brazilian multicentric prospective cohort, a subgroup of the SAFER study (Safety and Efficacy on COVID-19 Vaccine in Rheumatic Disease). All patients met international classification/diagnostic criteria for their respective diseases. Studied vaccines were inactivated SARS-CoV-2 (CoronaVac), adenoviral vectored (ChAdOx1/AstraZeneca and Ad26.COV2-S/Janssen), and mRNA (BNT162b2/Pfizer–BioNTech). The measurement of serum IgG levels against SARS-CoV-2 spike protein receptor-binding domain (IgG-RBD) using a chemiluminescence test (Abbott-Laboratories, IgG II Quant assay) assessed the immunogenicity. We collected serum samples at baseline and 28 days after each vaccine dose, with simultaneous assessment of disease activity scores, COVID-19 infections, and adverse events.
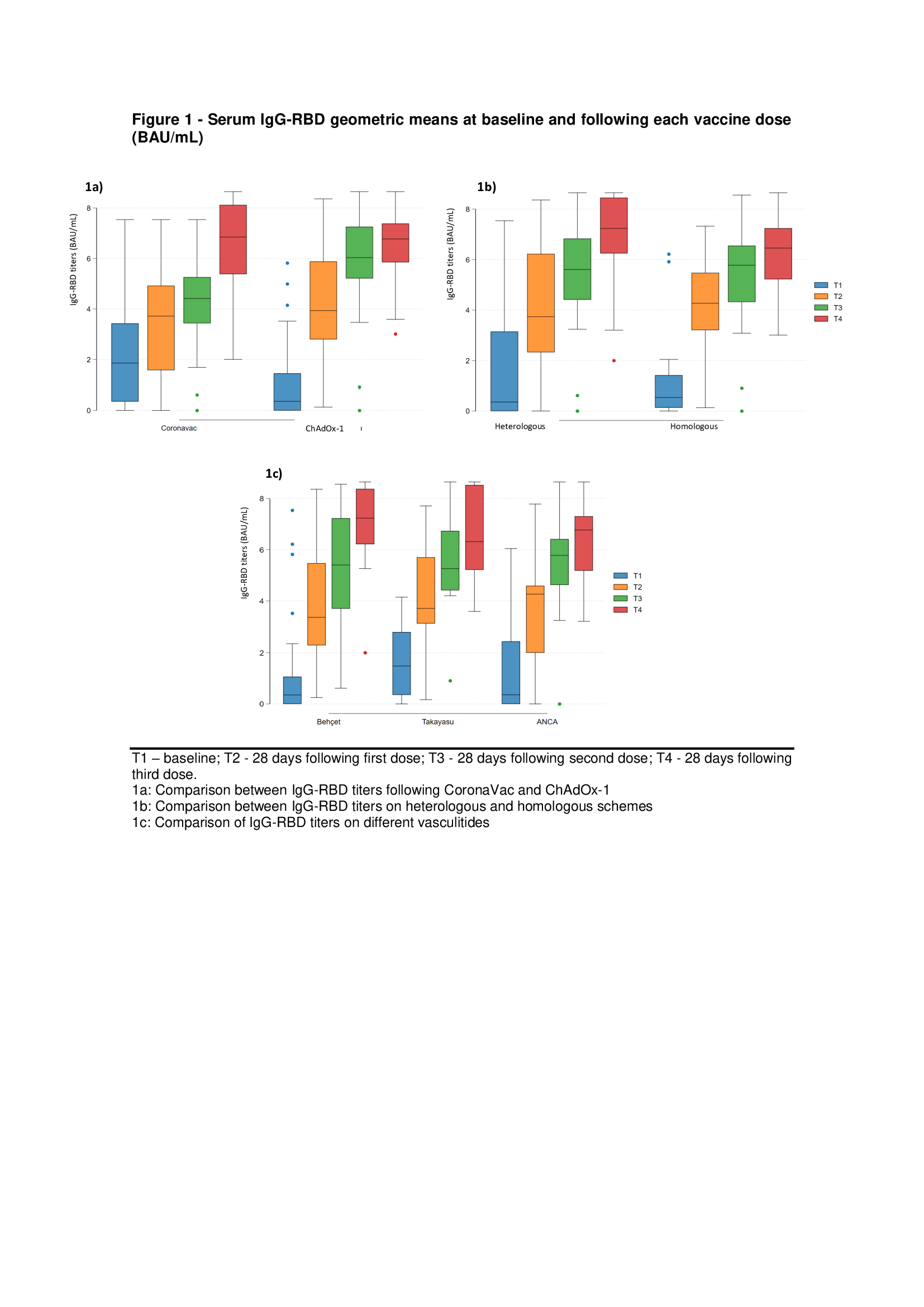
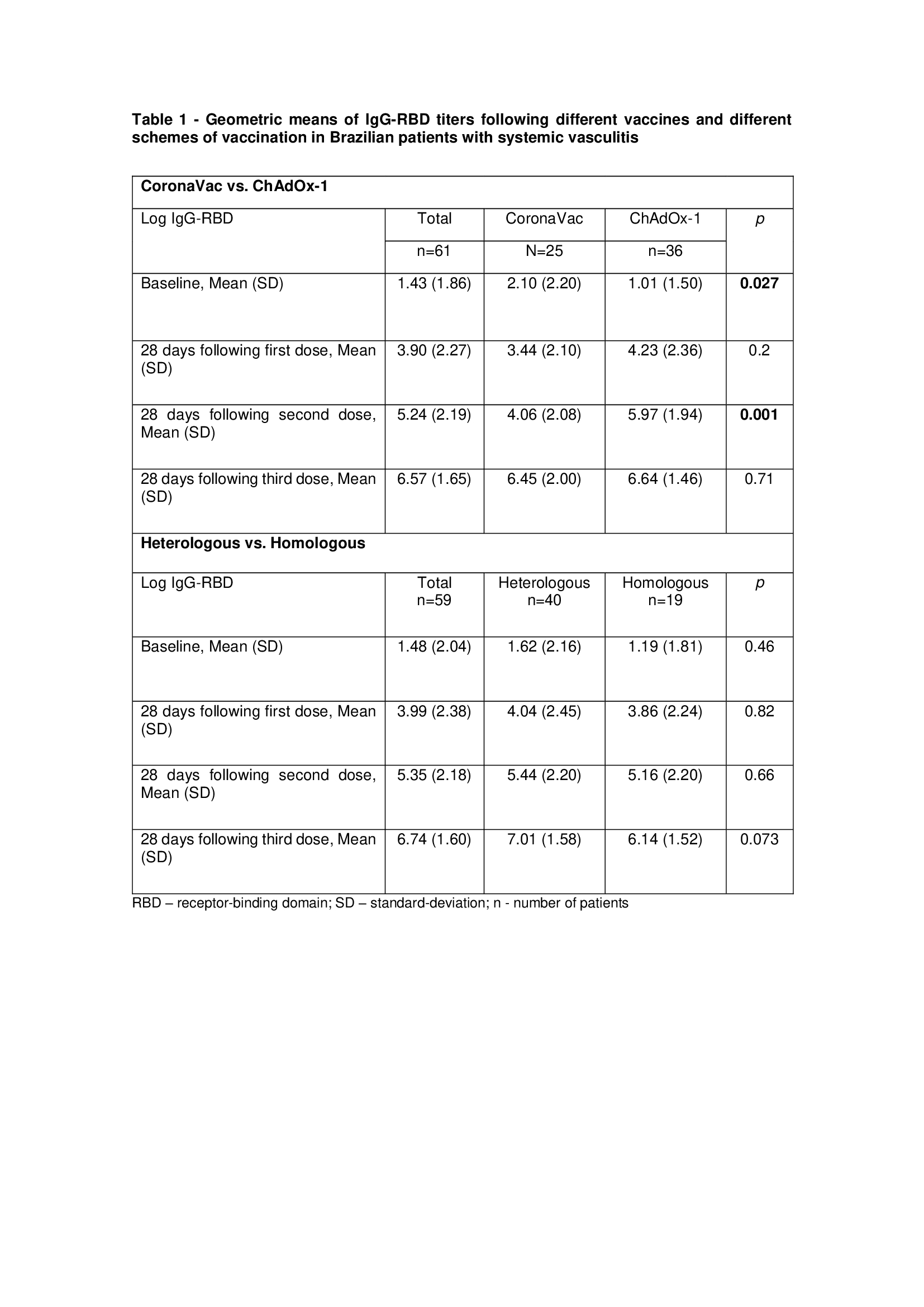
Results: Seventy-four patients received a single vaccine dose, 65 received two doses, and 59 completed the full vaccination regimen. Most patients received ChAdOx-1 (n = 36) or CoronaVac (n = 25) for the first two doses. The most administered booster dose was Pfizer–BioNTech. Behçet’s disease (BD), Takayasu arteritis, and antineutrophil cytoplasmic antibody (ANCA) associated vasculitis were the most frequent diagnosis in this cohort. Up to 49% of the patients had no comorbidities. Notably, none of the patients received Rituximab at baseline. ChadOx-1 achieved higher antibody titers than CoronaVac after two doses (p=0.002), but this difference disappeared after the booster dose (Table 1). Seropositivity rates tended to be higher in the heterologous vaccine group compared to the homologous three-dose scheme (Table 1). Immunogenicity was similar across different forms of vasculitis (Figure 1-c). No increase in disease relapse rates was observed in any form of vasculitis. No severe relapses or serious adverse events were reported.
Conclusion: In this Brazilian multicentric prospective cohort, the booster dose elicits a similar immune response in all patient groups, despite the initial difference in IgG-RBD titers after two doses of ChAdOx-1 and CoronaVac. Although a heterologous vaccine regimen showed a potential trend towards a more robust humoral response, this was not statistically significant, likely due to sample size limitations. Notably, the three-dose scheme was safe for all systemic vasculitides, with no increased disease activity observed.
To cite this abstract in AMA style:
Biegelmeyer E, Freitas de Aguiar M, Dias Cardoso Ribeiro P, Libardi Lira Machado K, da Penha Gomes Gouveia M, Paiva França Telles C, Basualto Dias S, Sarzi Sartori N, Karnopp T, de Oliveira Magalhães V, Matos Melo Campos Peixoto F, Hombre Dias L, Marques Veghini D, Vieira de Rezende R, Lino Baptista K, Guedes de Melo A, Alves Cruz V, Corrêa M, Kakehasi A, Rodrigues de Abreu Vieira R, Azevedo V, Assis Martins-Filho O, de Moura Castro C, Xavier R, Teixeira-Carvalho A, de Souza V, Monticelo O, Pinheiro M, Torres dos Reis Neto E, Sato E, Ferreira G, Pileggi G, Valim V, Silva de Souza A. Effectiveness of Three Doses of SARS-CoV-2 Vaccines in Brazilian Patients with Systemic Vasculitides: Preliminary Results of a Real-life Prospective Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-three-doses-of-sars-cov-2-vaccines-in-brazilian-patients-with-systemic-vasculitides-preliminary-results-of-a-real-life-prospective-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-three-doses-of-sars-cov-2-vaccines-in-brazilian-patients-with-systemic-vasculitides-preliminary-results-of-a-real-life-prospective-cohort/