Session Information
Date: Tuesday, November 14, 2023
Title: (2387–2424) Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The only steroid sparing agent approved for treatment of Giant Cell Arteritis (GCA) is the anti-interleukin-6 receptor antagonist tocilizumab. There remains uncertainty regarding treatment duration of tocilizumab and the optimal approach to medication withdrawal.
Methods: Patients who met the 2022 ACR/EULAR GCA classification criteria with a clinical diagnosis of GCA were prospectively enrolled. As per ACR/ Vasculitis Foundation guidelines all patients with a new diagnosis of GCA were started on tocilizumab if there were no contraindications. All patients had a 26-week glucocorticoid taper as per the GiACTA protocol. Tocilizumab was administered weekly for the first 12 months, and then every-other-week for an additional 12 months. Relapse of disease on tocilizumab was managed with temporary increases in systemic glucocorticoids.
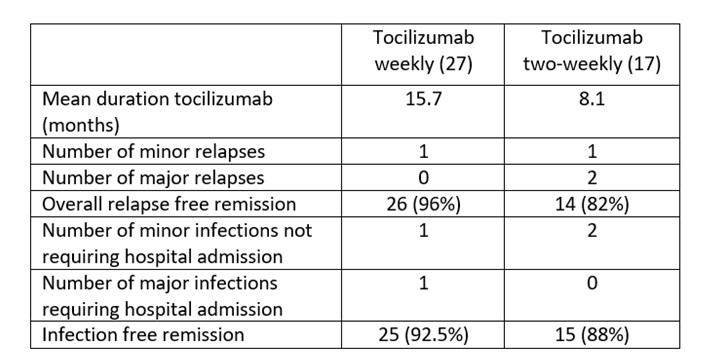
Results: 55 patients with newly-diagnosed GCA were included. Of these, 27 patients did not receive tocilizumab treatment for a variety of reasons; recent or active treatment for malignancy (7), history of diverticulitis (3), patient preference not to start (5), co-existing inflammatory condition for which other biologic treatment was preferred (1), patient frailty and/or recurrent infections (9), death before start of tocilizumab (1). 1 further patient only had one month of treatment before discontinuation for a drug reaction. 27 patients (49%) had weekly treatment with tocilizumab for 1 year. Of these only 17 had every second week tocilizumab at one year. 5 remained on a weekly dose due to previous visual loss and concern about preserving the remaining eye. 2 had other coexisting inflammatory diseases for which weekly dosing was required. 1 died of natural causes before dose spacing, and 2 stopped tocilizumab before spacing due to side effects. 27 patients had tocilizumab weekly for a mean of 15.7 months and 17 of had tocilizumab every-other-week at one year for a mean of 8.1 months. 2 patients (11.7%) had a major relapse on every-other-week tocilizumab (one aortic dissection and one with monocular visual loss). There were two minor relapses, one in the weekly dosing group and one in the two-weekly dosing group.
While on weekly tocilizumab, 1 patient had a minor infection treated in the community and 1 had a major infection (diverticulitis) requiring hospital admission. In the every-other-week tocilizumab group, two patient had minor infections treated in the community and no patient had a major infection requiring hospitalisation.
Conclusion: In a real-world prospective study cohort, only 49% (27/55) of patients were eligible for tocilizumab therapy (with GIACTA protocol steroid). 17/27 were eligible for dose reduction to every other week with 2/17 (11.7%) having major relapses of GCA at 24 months. In a real-life cohort tocilizumab therapy was only possible in 1 in 2 patients and when initiated was continued beyond the recommended period of 12 months in the majority of patients. Overall, 96% of patients in the weekly dosing group had relapse-free remission compared to 82% in the every-other-week treatment group. A dose reduction in tocilizumab after 12 months of weekly treatment maintained most patients in remission, had comparable safety outcomes and was cost effective.
To cite this abstract in AMA style:
Cowley S, Kirby C, Harkins P, Conway R, Murphy G, Kane D. Effectiveness of Dose Spacing with Tocilizumab in Giant Cell Arteritis Treatment [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-dose-spacing-with-tocilizumab-in-giant-cell-arteritis-treatment/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-dose-spacing-with-tocilizumab-in-giant-cell-arteritis-treatment/