Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Treatment (tx) guidelines for PsA recommend biologic DMARDs (bDMARDs) or targeted synthetic (ts) DMARDs after inadequate response to conventional synthetic DMARDs (csDMARDs)1. In clinical studies, Ixekizumab (IXE) has shown efficacy in patients (pts) with PsA who were bDMARD-naive2, TNFi-experienced3, and with and without concomitant csDMARDs4. Data from real-world studies is limited. This interim analysis reports the effectiveness of IXE and other b/tsDMARDs in b/tsDMARD-naive (naive) and -experienced (exp) pts as well as in monotherapy (mono) and in combination (comb) with any csDMARD at 12 months (M) in real-world setting
Methods:
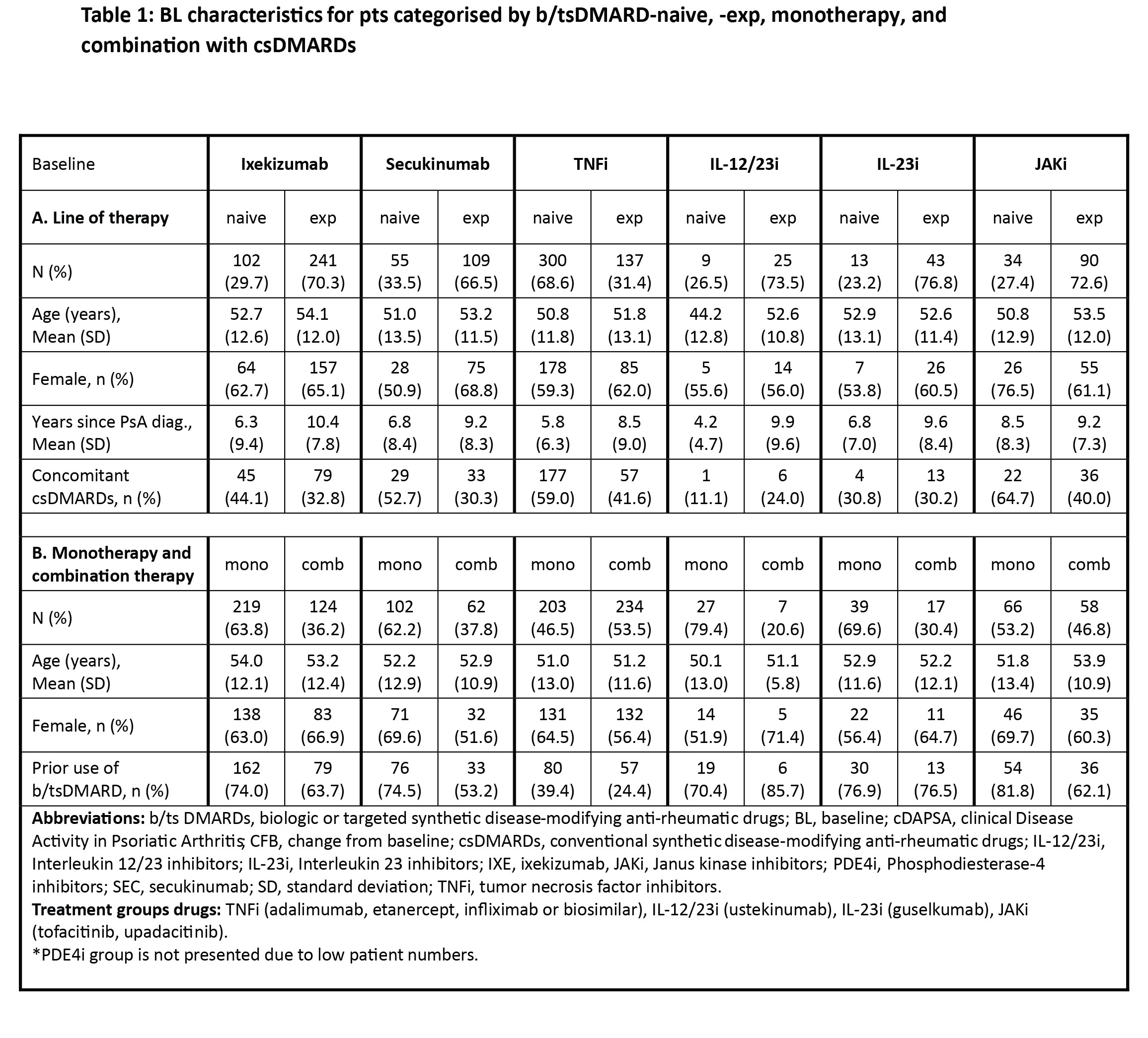
In PRO-SPIRIT, pts with PsA who initiated or switched to new b/tsDMARDs were evaluated in 5 European countries, and Canada. Pts were categorised by prior b/tsDMARD tx and concomitant csDMARD use at baseline (BL), respectively. Descriptive data for the analysis population at 12 M are presented. Mixed models for repeated measures (MMRM) were used to assess change from BL (CFB). Missing data were handled using multiple imputation.
Results: Of 1192* pts, TNF inhibitors (TNFis) (68.6%) and secukinumab (SEC) group (33.5%) had the highest proportion of naive pts, whereas TNFi (53.5%) and JAKi (46.8%) had the highest proportion of comb pts. At 12 M, similar mean CFB was observed in pts treated with IXE for clinical Disease Activity in Psoriatic Arthritis (cDAPSA) in the naïve (-13.6), exp (-12.1), mono (-12.3), and comb (-12.4) subgroups (subsequently reported in that order, herein); as well as for body surface area (BSA) (-5.0), (-3.6) (-4.4) and (-3.9). Similar trends were observed in tender joint counts and swollen joint counts. However, mean CFB in cDAPSA was lower in exp versus (vs) naive pts treated with SEC ( -8.9 vs –12.8), IL-12/23i (-7.4 vs -16.2) and IL-23i (-10.1 vs -17.2) and lower in mono vs combo in TNFi (-12.5 vs -15.2) and IL-23i (-11.0 vs -12.6). Mean CFB in BSA was lower in exp vs naive pts treated with TNFi (-2.8 vs -5.4), and IL-23i (-2.0 vs -5.3).
Conclusion: In real-world setting, IXE demonstrated similar effectiveness on joints and skin regardless of therapy line and concomitant csDMARDs, confirming findings from IXE clinical trials 2-4. Other treatments showed less consistent results either on exp pts (SEC, IL-12/23i, IL-23i) or in mono (TNFi, IL-23i).
References:
1. Gossec L, et al. Ann Rheum Dis. 2020;79:700-712.
2. Nash P, et al. Lancet. 2017;389:2317-2327
3. Mease P, et al. Ann Rheum Dis. 2017;76:79-87
4. Coates L, et al. Clin Rheum. 2022;41:3035–3047
To cite this abstract in AMA style:
Sewerin P, Gullick N, Russ H, Jing Ng K, O’Neill m, Moyano S, Giurdanella F, Gallego- Flores A, Ciccia F. Effectiveness of B/tsDMARDs Including Ixekizumab Per Line of Therapy and Concomitant CsDMARDs in Psoriatic Arthritis: Real-World Data from a Prospective Observational Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-b-tsdmards-including-ixekizumab-per-line-of-therapy-and-concomitant-csdmards-in-psoriatic-arthritis-real-world-data-from-a-prospective-observational-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-b-tsdmards-including-ixekizumab-per-line-of-therapy-and-concomitant-csdmards-in-psoriatic-arthritis-real-world-data-from-a-prospective-observational-study/