Session Information
Date: Saturday, November 16, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Giant Cell Arteritis (GCA) and Polymyalgia Rheumatica (PMR) are different manifestations of the same disease process. GCA is a vasculitis that affects large and medium-sized arteries in the head and neck, while PMR is an inflammatory disorder involving the shoulder and hips characterized by muscle aches and morning stiffness. Both conditions are treated with high-dose glucocorticoids which can cause adverse effects. Glucocorticoid-sparing agents like Tocilizumab help reduce glucocorticoid doses. The recommendations for the use of Tocilizumab still need to be fully established. This meta-analysis serves a crucial role in understanding the existing data on the effectiveness and safety of Tocilizumab in treating GCA and PMR
Methods: We searched databases, including PubMed, Ovid, and Embase, for studies on Tocilizumab in GCA and PMR, up to June 2024. The primary outcome was the relapse rate at week 52. Secondary outcomes included the cumulative glucocorticoid dose, medium and long-term relapse rates, time to relapse, short-term relapse rate, severe relapses, and safety outcomes. Pooled odds ratios (OR) and standardized mean differences (SMD) with 95% confidence intervals (CI) were calculated using a random-effects model.
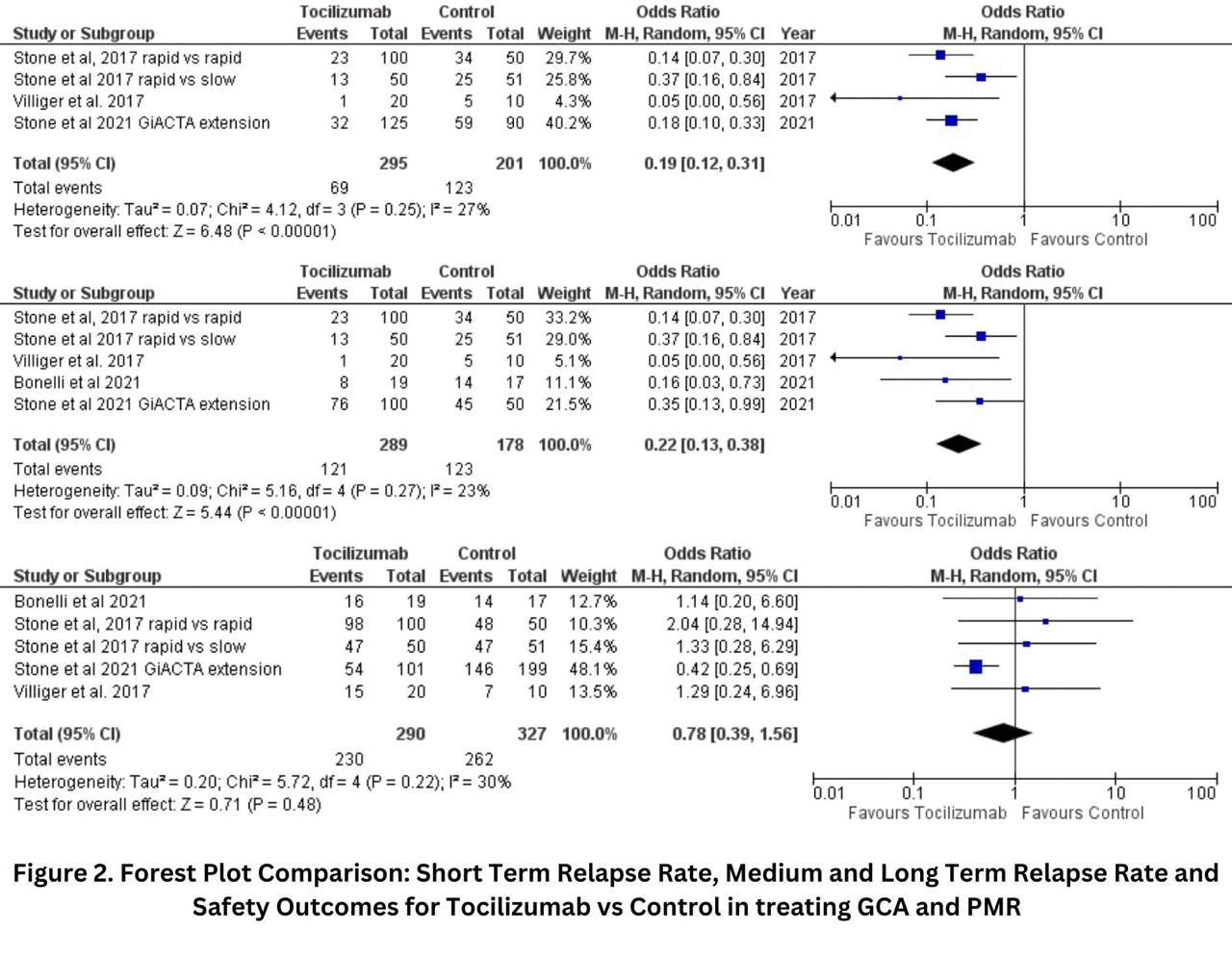
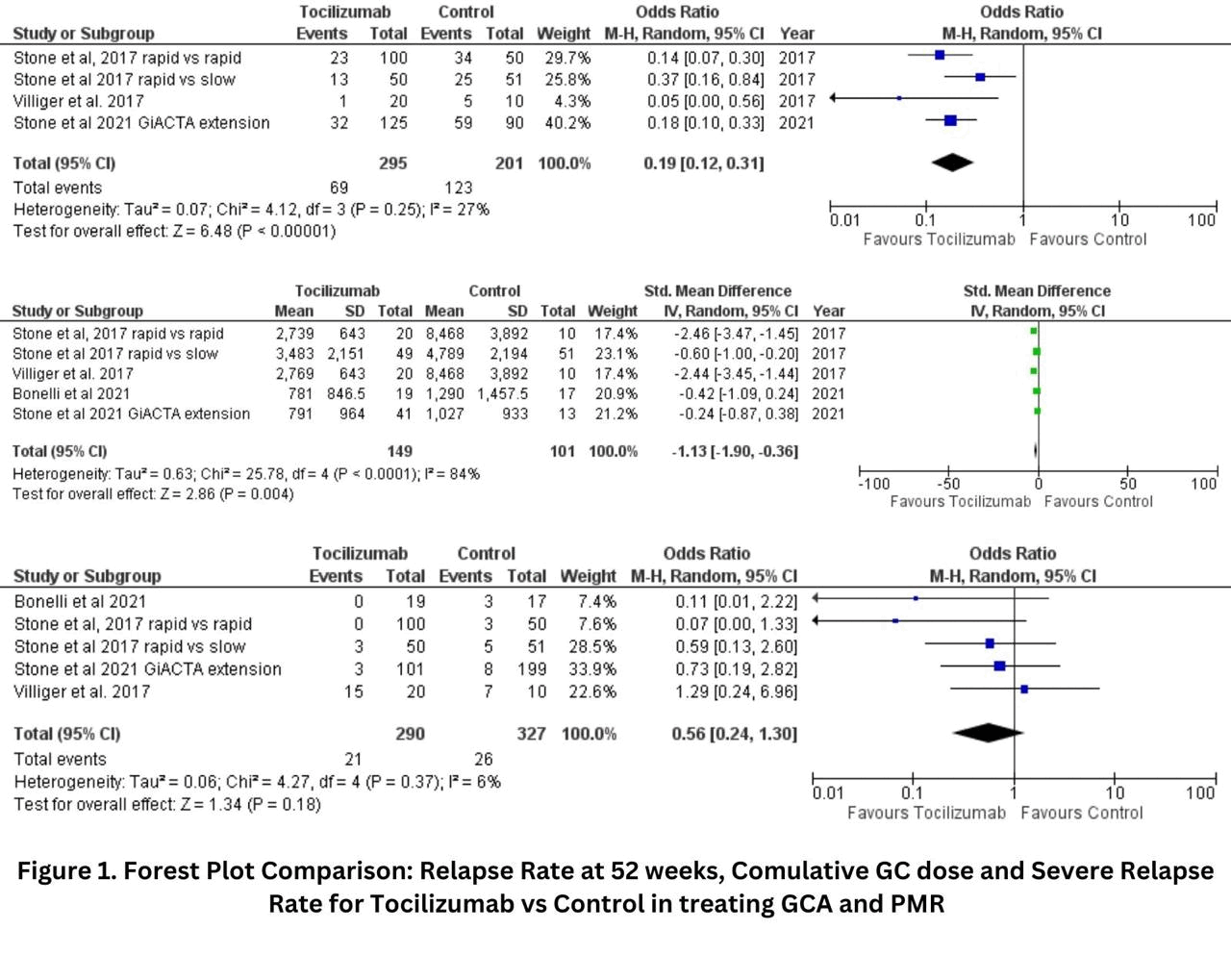
Results: We analyzed 5 studies with 617 patients: 290 patients in the tocilizumab group and 327 in the control group. All randomized controlled trials (RCTs) and observational studies reported a statistically significant reduction in relapse rates with tocilizumab compared to control groups. The short-term relapse rate showed a pooled odds ratio (OR) of 0.22 (95% CI: 0.13 to 0.38), indicating a significantly lower relapse rate in patients treated with tocilizumab compared to controls (I²=23%; p< 0.00001). The relapse rate at 52 weeks was also significantly lower in the tocilizumab group, with a pooled OR of 0.20 (95% CI: 0.11 to 0.35; I²=26%; p< 0.00001). The medium and long-term relapse rates showed a statistically significant difference, with a pooled OR of 0.19 (95% CI: 0.12 to 0.31; I²=27%; p< 0.00001). The severe relapses were reduced, but the results were not statistically significant, with a pooled OR of 0.56 (95% CI: 0.24 to 1.30; I²=6%; p=0.18). Additionally, tocilizumab significantly reduced the cumulative glucocorticoid dose, with a standardized mean difference (SMD) of -1.13 (95% CI: -1.90 to -0.36; I²=84%; p=0.004). Safety outcomes, including adverse events, showed no significant difference between tocilizumab and control groups, with a pooled OR of 0.78 (95% CI: 0.39 to 1.56; I²=30%; p=0.48). Overall, the findings indicate that tocilizumab significantly reduces relapse rates and glucocorticoid use in GCA and PMR patients, with no significant increase in adverse events compared to control treatments.
Conclusion: This meta-analysis provides robust evidence that Tocilizumab significantly reduces relapse rates and glucocorticoid use in patients with GCA and PMR. Importantly, Tocilizumab does not significantly increase adverse events compared to control treatments. These findings strongly support the use of Tocilizumab as an effective and safe therapeutic option for managing GCA and PMR.
To cite this abstract in AMA style:
Khan A, Shafique N, Qadeer A, Shaukat z. Effectiveness and Safety of Tocilizumab in the Treatment of Polymyalgia Rheumatica: A Meta-Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-tocilizumab-in-the-treatment-of-polymyalgia-rheumatica-a-meta-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-tocilizumab-in-the-treatment-of-polymyalgia-rheumatica-a-meta-analysis/