Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: CT-P13 is approved as a biosimilar of innovator infliximab for marketing in 81 countries. After approval, observational study has been conducted in Republic of Korea in patients with Rheumatoid Arthritis (RA), Ankylosing Spondylitis (AS), Psoriatic Arthritis (PsA) and Plaque Psoriasis (PS). The objective of this study is to evaluate the effectiveness and safety of CT-P13 under routine care.
Methods: This study included both biologic naïve patients (Naïve group) and patients who switched from other anti-tumor necrosis factor (TNF)s such as infliximab, adalimumab, golimumab and etanercept to CT-P13 (Switch group). Effectiveness was evaluated based on remission (DAS28<=2.6 in RA, BASDAI<3 in AS and absence of swollen and tender joint counts in PsA) and response (BASDAI 20/50/70 in AS and PASI 50/75 in PS). Adverse events (AEs) were collected over 6 months.
Results: Total 940 patients (RA 400, AS 531, PsA 3 and PS 6) were registered and 338 (36.0%) patients (RA 108, AS 228, PS 2) who switched to CT-P13 were included.
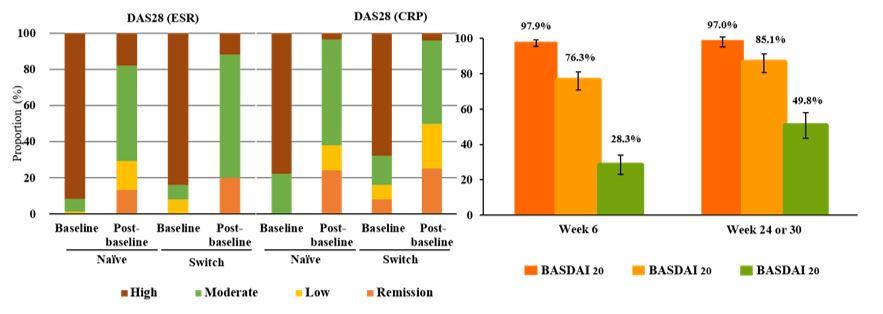
The proportion of patients achieving remission was similar between Naïve and Switch groups in both RA and AS during post-baseline visits (Table 1). In RA, the proportion of patients achieving each disease activity category by DAS28 was similar between Naïve and Switch groups (Figure 1). The proportion of patients who achieved BASDAI 20/50/70 response gradually increased from week 6 to week 24 or 30 in AS Naïve group (Figure 1).
Fifty percent of 2 naïve patients in PsA achieved remission. The proportions of both PASI 50 and 75 response were 50% at Week 22 in Naïve and 100% and 50% in Switch group, respectively during post-baseline visits in PS.
Throughout this study, treatment-emergent adverse events (TEAE) and treatment-emergent serious adverse events (TESAE) were reported as Table 2. Only 11% of patients experienced infection.
Table 1. Clinical remission in RA and AS
|
|
Naïve |
Switch |
|||
|
Baseline |
Post-baseline |
Baseline |
Post-baseline |
||
|
RA |
DAS28 (ESR) |
0/181 (0.0%) |
24/182 (13.2%) |
0/25 (0.0%) |
5/25 (20.0%) |
|
DAS28 (CRP) |
0/180 (0.0%) |
43/179 (24.0%) |
2/25 (8.0%) |
6/24 (25.0%) |
|
|
AS |
BASDAI |
2/292 (0.7%) |
199/292 (68.2%) |
112/209 (53.6%) |
150/210 (71.4%) |
Table 2. Summary of safety results
|
n/N (%) |
RA |
AS |
PsA |
PS |
|
TEAE |
198/400 (49.5) |
183/531 (34.5) |
1/3 (33.3) |
3/6 (50.0) |
|
TEAE related to CT-P13 |
73/400 (18.3) |
64/531 (12.1) |
0/3 (0.0) |
2/6 (33.3) |
|
TESAE |
52/400 (13.0) |
14/531 (2.6) |
0/3 (0.0) |
1/6 (16.7) |
|
TESAE related to CT-P13 |
15/400 (3.8) |
6/531 (1.1) |
0/3 (0.0) |
0/6 (0.0) |
|
Infusion-related reactions |
28/400 (7.0) |
11/531 (2.1) |
0/3 (0.0) |
0/6 (0.0) |
Figure 1. Clinical responses in RA and Naïve AS Patients
Conclusion: CT-P13 is efficacious and well-tolerated in RA/AS/PsA/PS patients. Efficacy and safety results in patients treated with CT-P13 were clinically consistent to historical data [1] [2] [3]. Especially, Switch group results showed that CT-P13 provides a useful alternative to other anti-TNFs.
References: [1] Kobayashi et al (2016)
[2] Hetland et al (2005)
[3] Hetland et al (2010)
To cite this abstract in AMA style:
Kim DW, Kim TH, Kwon SR, Lee EY, Son CN, Kim YS, Kim SH, Park YB, Hur JW, Lee HS, Lee SJ, Suh JH. Effectiveness and Safety of CT-P13 in Patients with Rheumatoid Arthritis, Ankylosing Spondylitis, Psoriatic Arthritis and Plaque Psoriasis: Observational Study in Republic of Korea [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/effectiveness-and-safety-of-ct-p13-in-patients-with-rheumatoid-arthritis-ankylosing-spondylitis-psoriatic-arthritis-and-plaque-psoriasis-observational-study-in-republic-of-korea/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-ct-p13-in-patients-with-rheumatoid-arthritis-ankylosing-spondylitis-psoriatic-arthritis-and-plaque-psoriasis-observational-study-in-republic-of-korea/