Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Observational data on the use of secukinumab for the treatment of spondyloarthritides are still lacking. The aim of this study is to evaluate the effectiveness and the 3-year retention rate of secukinumab in psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA) patients in a real-life setting.
Methods: Data of all PsA and axSpA patients (diagnosed according to CASPAR and ASAS criteria, respectively) treated with secukinumab were prospectively collected in the Italian multicentric LORHEN registry. Effectiveness was measured as the mean change from baseline of Disease Activity in PSoriatic Arthritis score (DAPSA) in PsA and Ankylosing Spondylitis Disease Activity Score (ASDAS) in axSpA patients. Rates of DAPSA remission and ASDAS inactive disease were also computed. The 3-year retention rate was calculated by the Kaplan-Meier method and compared between PsA and axSpA by a log-rank test. A descriptive analysis of reasons for discontinuation was performed.
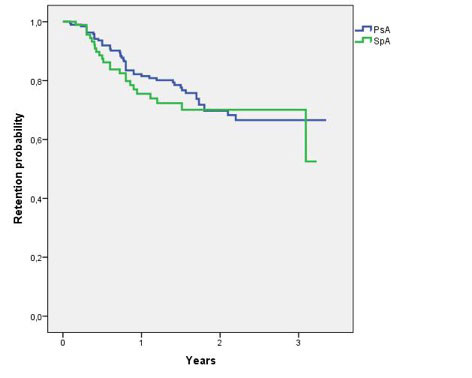
Results: The study population included 195 PsA (55.4% females, mean age 50.7 [±11.8] years, mean disease duration 10 [±7.8] years, mean baseline DAPSA 23.12 [±12.3]) and 94 axSpA (61.7% males, mean age 49.1 [±12.7] years, mean disease duration 10.4 [±9.4] years, mean baseline ASDAS 3.41 [±1.1]) patients who received secukinumab as first (26.5 and 33%, respectively) or subsequent biologic agent. Compared with baseline, the 3-, 6- and 12-month mean values of both DAPSA (12.6 [±9], 11.2 [±10.5] and 9.3 [±7.5], respectively) and ASDAS (2.23 [±0.9], 2.15 [±0.9], and 1.84 [±0.9], respectively) were significantly decreased (p< 0.001 for all the timepoints). The 3-, 6-, and 12-month rates of remission/inactive disease were 15.5, 25.4, and 30.5% in PsA and 18, 23.7, and 28.6% in axSpA group, respectively. One- and 3-year retention rate (figure 1) were respectively 79.4% and 66.6% in PsA and 72.3% and 70.1% in axSpA patients, with no significant difference between the two groups (p=0.517). The most frequent reason for withdrawal was inefficacy in both PsA (n=41) and axSpA (n=20), whereas only 8 PsA and 6 axSpA patients discontinued secukinumab because of adverse events.
Conclusion: Our real-life data confirmed the effectiveness and the favorable safety profile of secukinumab for both PsA and axSpA, resulting in a high 3-year retention rate.
To cite this abstract in AMA style:
Favalli E, Marchesoni A, Balduzzi S, Montecucco C, Lomater C, Crepaldi G, Tamanini S, Bazzani C, Fusaro E, Priora M, Ianniello A, Caporali R. Effectiveness and Retention Rate of Secukinumab for Psoriatic Arthritis and Axial Spondyloarthritis: Real-life Data from the Italian LORHEN Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effectiveness-and-retention-rate-of-secukinumab-for-psoriatic-arthritis-and-axial-spondyloarthritis-real-life-data-from-the-italian-lorhen-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-retention-rate-of-secukinumab-for-psoriatic-arthritis-and-axial-spondyloarthritis-real-life-data-from-the-italian-lorhen-registry/