Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: SURPASS, a phase 3b randomized controlled study in patients with radiographic axial spondyloarthritis (r-axSpA), found low spinal radiographic progression over 2 years with no significant difference between the secukinumab (SEC) and adalimumab biosimilar (SDZ- ADL) treatment arms.1,2 Baseline (BSL) radiographic damage (presence of syndesmophytes) and elevated C-reactive protein (CRP) levels have been identified as predictors of radiographic progression in r-axSpA.3 This study evaluated the effect of SEC and SDZ-ADL on spinal radiographic progression in subgroups of patients based on the presence of syndesmophytes and elevated high-sensitivity CRP (hsCRP) levels at BSL, from the SURPASS study.
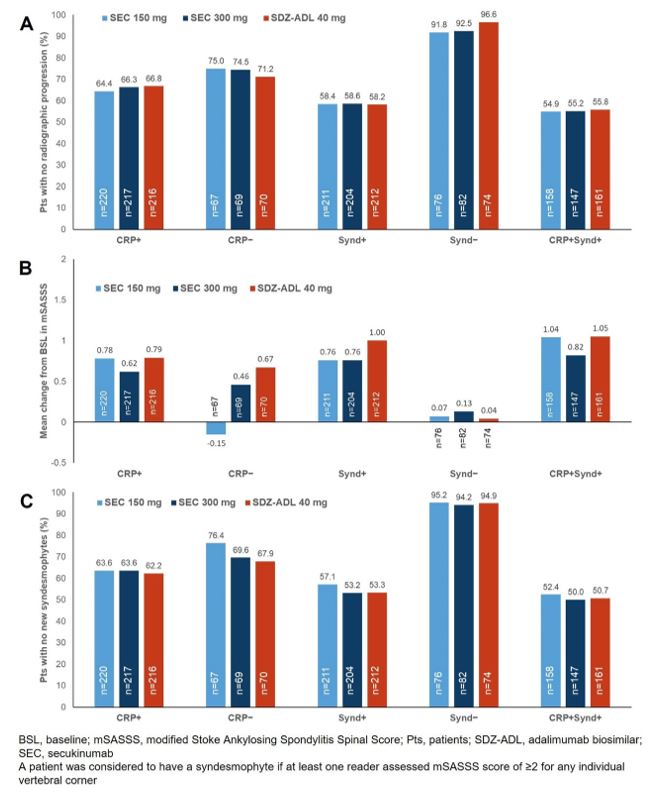
Methods: Biologic-naïve patients with active r-axSpA and with hsCRP ≥5 mg/L and/or ≥1 syndesmophyte(s) on spinal radiographs were randomized (1:1:1) to SEC (150 or 300 mg; dose-blinded) or SDZ-ADL (40 mg; open label). Patients were categorized into the following subgroups at BSL: hsCRP ≥5 mg/L (CRP+), hsCRP < 5 mg/L (CRP−), presence of syndesmophyte(s) (Synd+), absence of syndesmophyte(s) (Synd−), and CRP+Synd+. The proportion of patients with no radiographic progression (change from BSL in modified Stoke Ankylosing Spondylitis Spinal Score [mSASSS] ≤0.5), mean change from BSL in mSASSS, and proportion of patients with no new syndesmophytes(s) in each subgroup at week 104 (all as observed) are reported.
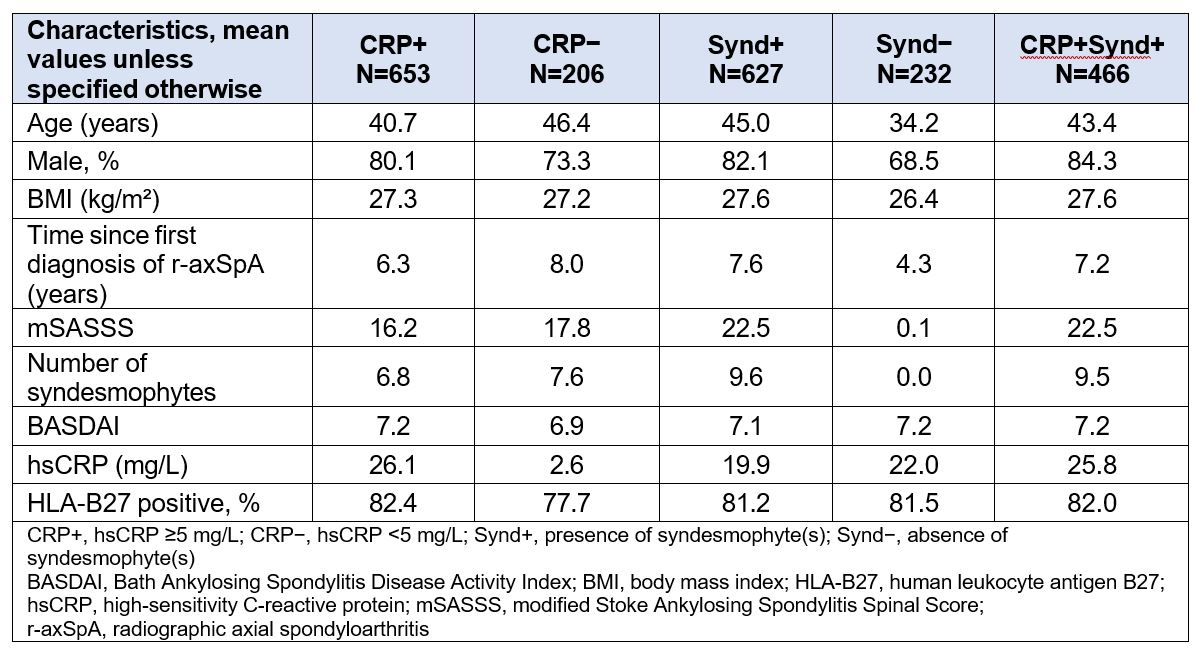
Results: Of the 859 patients, 653 (76%) were CRP+, 627 (73%) were Synd+, and 466 (54%) were CRP+Synd+ at BSL. Demographic and BSL disease characteristics were largely balanced across subgroups and treatment arms, except for the Synd− group in which mean age, proportion of male patients, and mean time since diagnosis were lower than in other subgroups (Table 1). All radiographic outcomes at week 104 were similar across treatment arms; however, differences were observed between subgroups irrespective of the treatment arm. The Synd− subgroup followed by the CRP− subgroup showed the least progression in all radiographic outcomes (as indicated by the higher proportion of patients with no radiographic progression and no new syndesmophytes, and lower mean change from BSL in mSASSS), across treatment arms (Figure 1). The CRP+Synd+ subgroup followed by the Synd+ subgroup and the CRP+ subgroup had higher radiographic progression compared with the Synd− and CRP− subgroups (Figure 1).
Conclusion: Spinal radiographic progression over 2 years was low with no notable difference between SEC and SDZ-ADL arms regardless of the presence or absence of specific predictive factors for progression (syndesmophytes/elevated CRP). Expectedly, patients from subgroups without predictive factors (especially Synd−, followed by CRP−) had lowest rates of radiographic progression. The presence of syndesmophytes is a stronger predictor than the presence of an elevated CRP.
References:
- Baraliakos X, et al. Clin Drug Investig. 2020;40(3):269-78
- Baraliakos X, et al. Arthritis Rheumatol. 2022;74 (suppl 9)
- Poddubnyy D, et al. Arthritis Rheum. 2012;64(5):1388-98
To cite this abstract in AMA style:
Baraliakos X, van der Heijde D, Machado P, Navarro-Compán V, Gensler L, Pertel P, Quebe-Fehling E, Readie A, Richards H, Poddubnyy D. Effect of Secukinumab versus Adalimumab Biosimilar on Radiographic Progression in Patients with Radiographic Axial Spondyloarthritis: Results from Subgroup Analyses by Baseline Syndesmophytes and C-reactive Protein Status [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effect-of-secukinumab-versus-adalimumab-biosimilar-on-radiographic-progression-in-patients-with-radiographic-axial-spondyloarthritis-results-from-subgroup-analyses-by-baseline-syndesmophytes-and-c-re/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-secukinumab-versus-adalimumab-biosimilar-on-radiographic-progression-in-patients-with-radiographic-axial-spondyloarthritis-results-from-subgroup-analyses-by-baseline-syndesmophytes-and-c-re/