Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Guselkumab (GUS), a selective IL-23p19 inhibitor, resulted in greater mean improvements in BASDAI scores vs placebo (PBO) at W24 among patients (pts) with active PsA and investigator-confirmed sacroiliitis in pooled post hoc analyses of data from two phase 3 trials, DISCOVER-1&2. Improvements in these symptoms of axial involvement were maintained through 1 year.1We aimed to further assess maintenance of GUS effect on symptoms of axial involvement among biologic-naïve PsA pts with investigator-confirmed sacroiliitis through 2 years of DISCOVER-2.
Methods: In this phase 3, double-blind, PBO-controlled study, 739 bio-naïve pts with active PsA (≥5 swollen joints, ≥5 tender joints, CRP ≥0.6mg/dL despite standard therapies) were randomized 1:1:1 and treated with GUS 100 mg every 4 weeks (Q4W; n=245), GUS 100 mg at W0, W4, then Q8W (n=248), or PBO (n=246), with PBOàGUS 100 mg Q4W at W24. Pts identified by the investigator as having axial symptoms and sacroiliitis (prior X-ray or MRI, or pelvic X-ray at screening) were evaluated. Efficacy was assessed by change in BASDAI, modified BASDAI (mBASDAI, excluding Q3 [peripheral joint pain]), and BASDAI Q2 (Spinal Pain) scores and proportions of pts achieving BASDAI 50 response, Spinal Pain score ≤2, and AS Disease Activity Score (ASDAS) responses through W100. Through W24, pts who met treatment failure criteria or had missing data were considered nonresponders or to have no change from baseline. After W24, missing data were imputed as nonresponse for binary endpoints or no change from baseline for continuous endpoints (nonresponder imputation [NRI]). Axial-related outcomes were also summarized by HLA-B27 status (+/-) among 149 pts with available data.
Results: 246 pts had sacroiliitis confirmed by the investigator. Baseline characteristics were similar across treatment groups (62% male; mean age 44.4 years); mean BASDAI scores ranged from 6.5-6.6. At W24, LSmean/mean changes in BASDAI (-2.4/-2.6) and ASDAS (-1.3/-1.5) scores were greater in GUS than PBO-treated pts. Mean changes from baseline were maintained through W100 in GUS-treated pts for BASDAI (-3.1), Spinal Pain (-3.1), mBASDAI (‑3.1), and ASDAS (‑1.7) scores. Similar response patterns were observed for BASDAI 50 response rates among GUS-treated pts (W24 38-40%; W100 49-54%). At W24, GUS-treated pts had higher response rates for achievement of ASDAS inactive disease, major improvement, and clinically important improvement vs. PBO; response rates (NRI) were maintained, or in some cases further increased, at 2 years. Consistent results were observed for achievement of ASDAS LDA and Spinal Pain score ≤2 (data not shown). GUS-related improvements in axial symptoms through W100 were generally consistent across pts who were HLA-B27+/- (data not shown).
Conclusion: In bio-naive pts with active PsA and investigator-confirmed sacroiliitis, GUS provided durable improvements in axial symptoms through W100, with substantial proportions of pts achieving and maintaining clinically meaningful improvements.
References:
1. Mease PJ. Lancet Rheumatol 2021; In press.
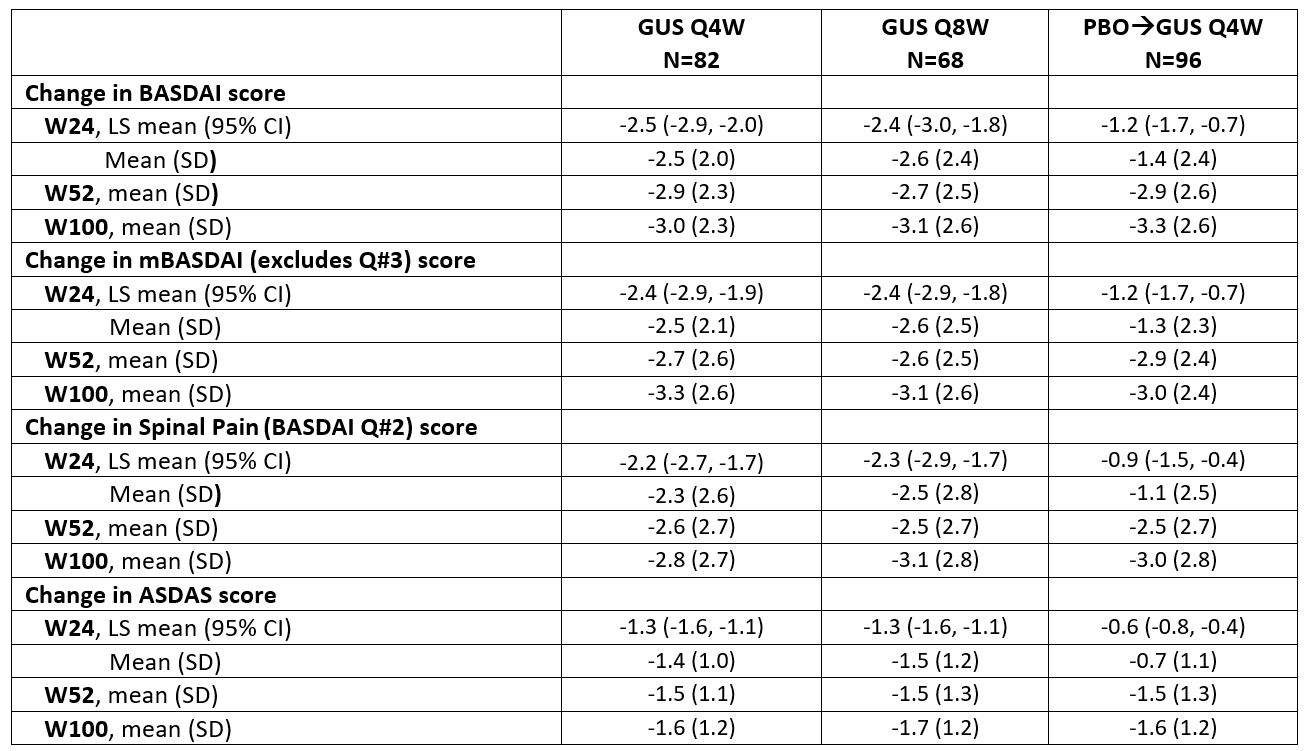 Table. Axial symptom assessments through W100 in PsA pts with investigator-confirmed sacroiliitis in DISCOVER_2 (NRI)
Table. Axial symptom assessments through W100 in PsA pts with investigator-confirmed sacroiliitis in DISCOVER_2 (NRI)
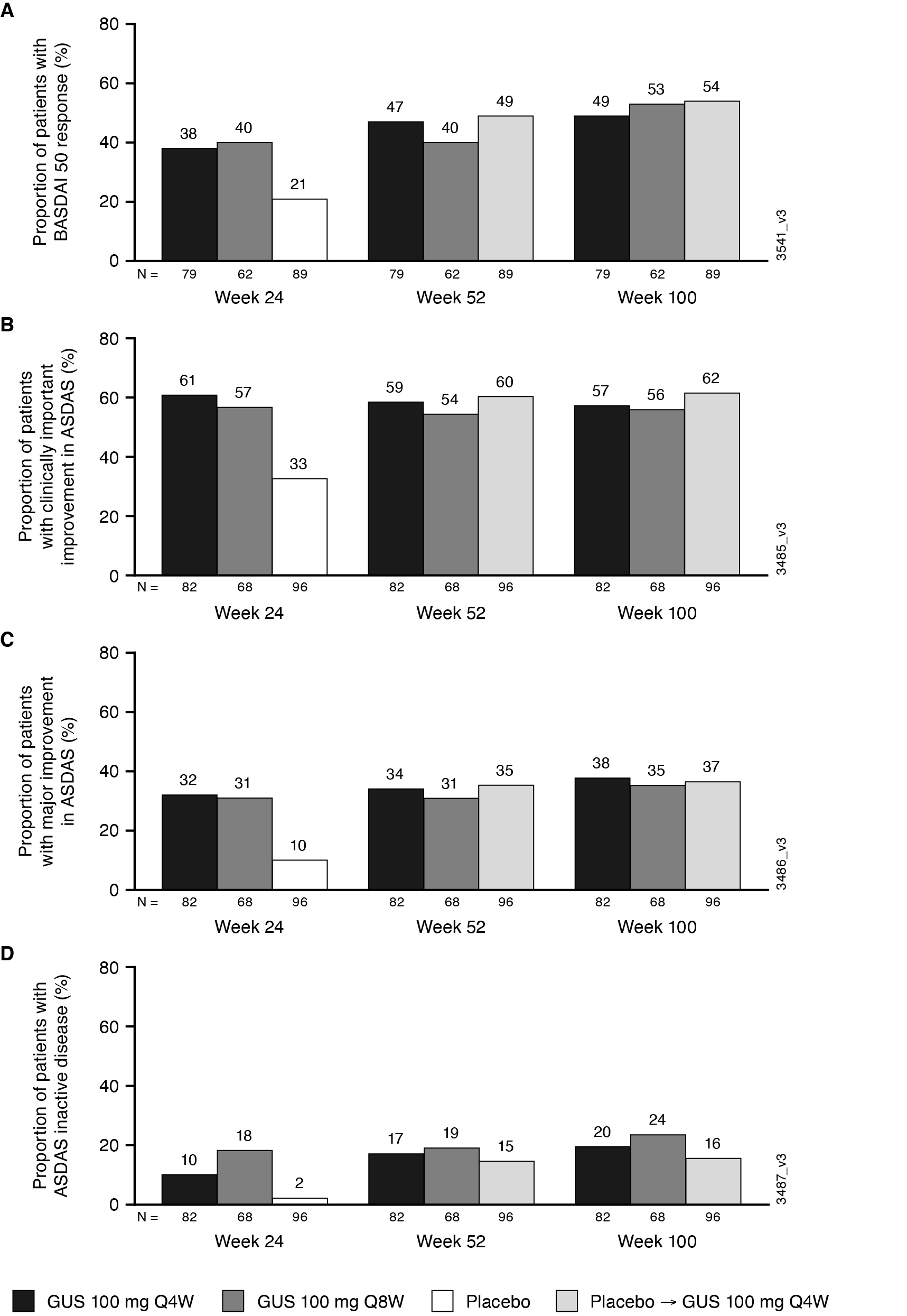 Figure. Proportion of PsA patients with investigator-confirmed sacroiliitis achieving BASDAI 50 response (A), and ASDAS clinically important improvement (decrease ≥1.1) (B), major improvement (decrease ≥2.0) (C), and inactive disease ( < 1.3) (D) through W100 (NRI)
Figure. Proportion of PsA patients with investigator-confirmed sacroiliitis achieving BASDAI 50 response (A), and ASDAS clinically important improvement (decrease ≥1.1) (B), major improvement (decrease ≥2.0) (C), and inactive disease ( < 1.3) (D) through W100 (NRI)
To cite this abstract in AMA style:
Mease P, Helliwell P, Gladman D, Poddubnyy D, Baraliakos X, Chakravarty S, Kollmeier A, Xu X, Sheng S, Xu S, Shawi M, van der Heijde D, Deodhar A. Effect of Guselkumab (TREMFYA®), a Selective IL-23p19 Inhibitor, on Axial-Related Endpoints in Patients with Active PsA: Results from a Phase 3, Randomized, Double-blind, Placebo-controlled Study Through 2 Years [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-guselkumab-tremfya-a-selective-il-23p19-inhibitor-on-axial-related-endpoints-in-patients-with-active-psa-results-from-a-phase-3-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-guselkumab-tremfya-a-selective-il-23p19-inhibitor-on-axial-related-endpoints-in-patients-with-active-psa-results-from-a-phase-3-randomized-double-blind-placebo-controlled-study/
