Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Glucocorticoid (GC)-induced osteoporosis (GIOP) remains the most common secondary cause of osteoporosis. Despite approved therapies, many subjects do not receive GIOP prevention or treatment. There is increased RANKL and decreased osteoprotegerin (OPG) expression in GIOP. Denosumab (DMAb) is a monoclonal antibody to RANKL. This study was designed to assess the safety and efficacy of DMAb compared with risedronate (RIS) in GC-treated individuals, in whom treatment guidelines advocate a GIOP intervention.
Methods: This was a phase 3, randomized, double-blind, active-controlled study to evaluate DMAb vs. RIS in GC-treated individuals for 24 mos. Eligible subjects were women and men ≥18 yrs receiving GC therapy at a dose ≥7.5 mg prednisone daily or its equivalent for ≥3 mos or <3 mos prior to screening (GC-continuing [GC-C] and GC-initiating [GC-I], respectively). All subjects <50 yrs were required to have a history of osteoporotic fracture. GC-C subjects ≥50 yrs were required to have a lumbar spine (LS), total hip (TH), or femoral neck bone mineral density (BMD) T‑score ≤‒2.0; or a T-score ≤‒1.0 with a history of fracture. Subjects were randomized 1:1 to SC DMAb 60 mg every 6 mos or oral RIS 5 mg daily for 24 mos. Subjects were to receive daily calcium (≥1000 mg) and vitamin D (≥800 IU) supplementation. The primary objective was to demonstrate in the GC-C and GC-I subpopulations separately that DMAb was not inferior to RIS with respect to percentage change from baseline in LS BMD at 12 mos. Secondary objectives were to assess superiority of DMAb over RIS with respect to percentage change from baseline in LS and TH BMD at 12 mos. The study remains blinded and is ongoing.
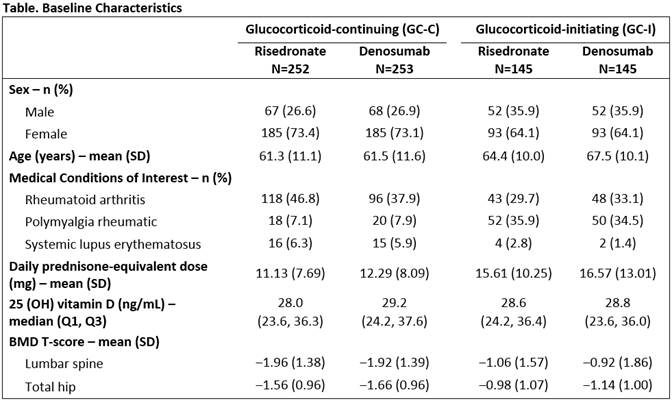
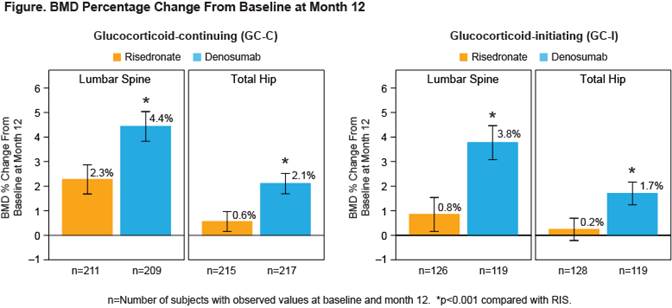
Results: A total of 795 subjects (505 GC-C and 290 GC-I) enrolled in the study. Baseline characteristics were balanced between treatment groups (Table). Non-inferiority and superiority with DMAb were demonstrated for both the GC-C and GC-I subpopulations, due to significantly greater gains in BMD compared with RIS at the LS and TH in both subpopulations (Figure). The incidences of adverse events (AEs) and serious AEs, including serious AEs of infection, as well as fracture, were similar between treatment groups and consistent with the known safety profile of DMAb.
Conclusion: DMAb significantly increased BMD more than RIS at the spine and hip at 12 months. The overall safety profile was similar between treatment groups. DMAb has the potential to become another treatment option for patients newly initiating or continuing GC who are at risk for fracture.
To cite this abstract in AMA style:
Saag K, Wagman R, Geusens P, Adachi J, Messina O, Emkey R, Chapurlat R, Daizadeh N, Pannacciulli N, Lems W. Effect of Denosumab Compared with Risedronate in Glucocorticoid-Treated Individuals: Results from the 12-Month Primary Analysis of a Randomized, Double-Blind, Active-Controlled Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/effect-of-denosumab-compared-with-risedronate-in-glucocorticoid-treated-individuals-results-from-the-12-month-primary-analysis-of-a-randomized-double-blind-active-controlled-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-denosumab-compared-with-risedronate-in-glucocorticoid-treated-individuals-results-from-the-12-month-primary-analysis-of-a-randomized-double-blind-active-controlled-study/