Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Certolizumab pegol (CZP), a PEGylated Fc-free anti-TNF, has shown efficacy in reducing signs and symptoms of psoriatic arthritis (PsA).1 RAPID-PsA (NCT01087788) is the first report of a biologic in PsA to include patients (pts) with prior anti-TNF exposure.
Methods:
The ongoing 158 week (Wk) RAPID-PsA trial is double-blind and placebo (PBO) controlled to Wk24, dose-blind to Wk48 and then open label to Wk158. Recruited pts had active PsA and had failed ≥1 DMARD. Pts could have experienced secondary failure to 1 previous anti-TNF. Pts were randomized 1:1:1 to PBO, or 400mg CZP at Wk 0, 2 and 4 (loading dose, LD) followed by either 200mg CZP every 2 wks (Q2W) or 400mg CZP every 4 wks (Q4W). Pts receiving PBO who failed to achieve a ≥10% decrease in tender and swollen joint counts at both Wks14 and 16 were rescued and randomized at Wk16 to receive CZP 200mg Q2W or CZP 400mg Q4W following LD. The clinical primary endpoint was ACR20 response at Wk12. Secondary outcomes measured in the study include PASI75, Leeds Enthesitis Index (LEI), Leeds Dactylitis Index (LDI) and Modified Nail Psoriasis Severity Index (mNAPSI). Non-responder imputation was used for ACR and PASI75; Last observation carried forward was used for LEI, LDI and mNAPSI.
Results:
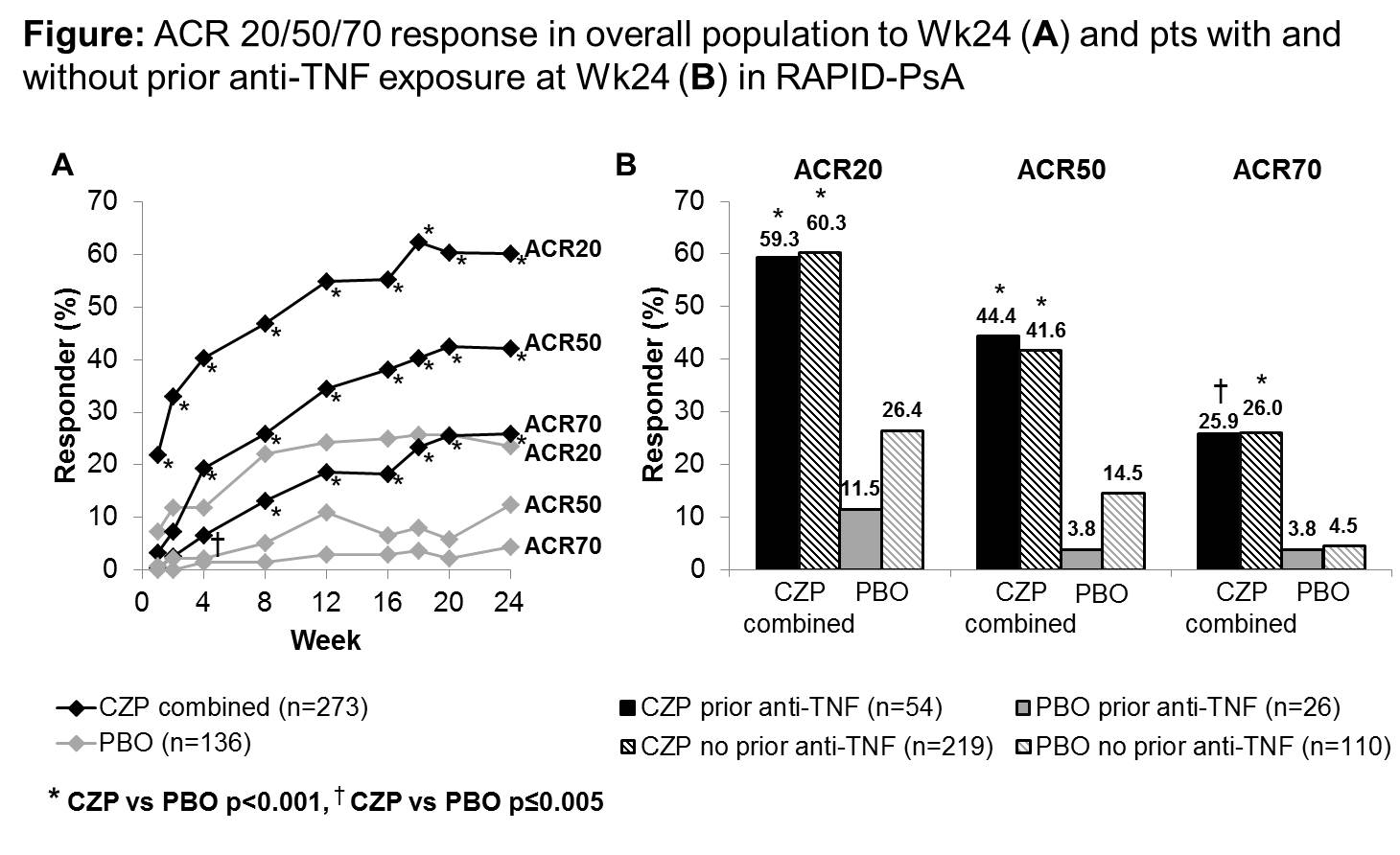
409 pts were randomized. Baseline (BL) demographics were similar between groups. 19.1% and 19.8% of PBO and CZP (combined dose) pts received prior anti-TNF. ACR20 response at Wk12 was significantly higher in the CZP 200 mg Q2W and CZP 400 mg Q4W arms vs. PBO (58.0% and 51.9% vs. 24.3% [p<0.001 for both]) and was observed as early as Wk1 (21.0% [p=0.001] and 23.0% [p<0.001] vs.7.4%).1 PASI75 response at Wk24 for pts with ≥3% psoriasis body surface area at BL (61.6% randomized set [RS]) was 62.2% with CZP 200mg Q2W and 60.5% with CZP 400mg Q4W vs 15.1% PBO (p<0.001 for both). In pts with BL enthesitis (64.3% RS) LEI change from BL at Wk24 was -2.0 with CZP 200mg Q2W (p<0.001) and -1.8 with CZP 400mg Q4W (p=0.003) vs -1.1 PBO. For pts with BL nail disease (73.3% RS) mNAPSI change from BL at Wk24 was -1.6 with CZP 200mg Q2W and -2.0 with CZP 400mg Q4W vs -1.1 PBO. No differences in LDI change from BL were observed in pts with BL dactylitis. At Wk24, ACR response rates were similar between CZP arms and greater vs. PBO irrespective of prior anti-TNF exposure (Figure). Adverse events (AEs) occurred in 62% vs 68% and SAEs in 7% vs. 4% in CZP vs PBO pts (combined dose), respectively. Two deaths occurred up to Wk24, one sudden death of unknown cause (CZP 400mg Q4W) and one myocardial infarct (CZP 200mg Q2W). No new safety signals were observed.
Conclusion:
Rapid improvements in the signs and symptoms of PsA, as well as skin manifestations and nail disease of psoriasis, were observed across both CZP dosing regimens. Similar ACR response rates with CZP were observed in pts with and without prior anti-TNF exposure.
Reference:
1. Mease P, Ann Rheum Dis 2012;71(Suppl3):150
Disclosure:
P. J. Mease,
UCB Pharma,
5,
UCB Pharma,
2,
UCB Pharma,
8;
R. Fleischmann,
UCB Pharma,
2,
UCB Pharma,
5;
J. Wollenhaupt,
UCB Pharma,
5,
UCB Pharma,
8;
A. A. Deodhar,
UCB Pharma,
2,
UCB Pharma,
5;
D. Kielar,
UCB Pharma,
1,
UCB Pharma,
3;
F. Woltering,
UCB Pharma,
1,
UCB Pharma,
3;
C. Stach,
UCB Pharma,
3;
B. Hoepken,
UCB Pharma,
3;
T. Arledge,
UCB Pharma,
3;
D. van der Heijde,
UCB Pharma,
5,
UCB Pharma,
2,
UCB Pharma,
8.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-certolizumab-pegol-on-signs-and-symptoms-in-patients-with-psoriatic-arthritis-with-and-without-prior-anti-tnf-exposure-24-week-results-of-a-phase-3-double-blind-randomized-placebo-controlle/