Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Open label studies have shown the efficacy of canakinumab (CAN), a fully human and highly specific anti-IL-1β monoclonal antibody, in patients (pts) with Periodic Fever Syndromes (PFS), which include colchicine-resistant Familial Mediterranean Fever (crFMF), Hyper-IgD Syndrome/Mevalonate Kinase Deficiency (HIDS/MKD) and TNF-Receptor Associated Periodic Syndrome (TRAPS). Currently, no data is available on the effect of CAN on Health-Related Quality of Life (HRQoL) in pts with PFS. The study objective was to assess the effect of CAN on HRQoL using Child Health Questionnaire-Parent Form 50 (CHQ-PF50) and Short Form-12 Health Survey (SF-12) in pts with PFS.
Methods: In this Phase 3, randomized, placebo-controlled study of CAN in pts with PFS (NCT02059291), SF-12 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were assessed in adults, whereas CHQ-PF50 Physical Summary (PhS) and Psychosocial Summary (PsS) scores were assessed in children (>5–<18 years).
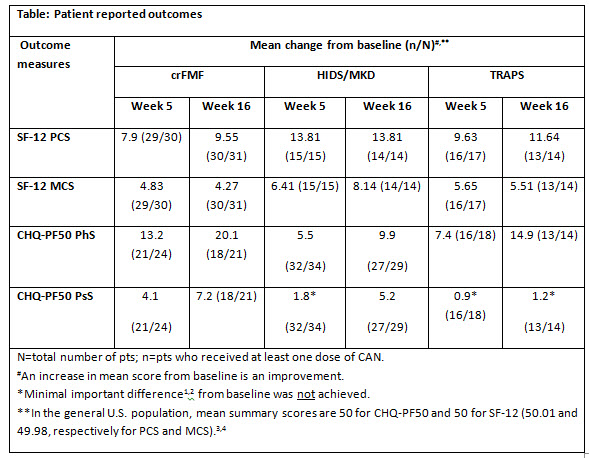
Results: Of the 181 pts (63 crFMF, 72 MKD/HIDS, 46 TRAPS) randomized to CAN or placebo, 71 were adults (age ≥18 years) and 110 were children (age ≥2–<18 years). Pts who initially received placebo and did not respond were switched to CAN. Treatment with CAN was associated with an early clinically meaningful improvement in SF-12 PCS scores reported at Week (Wk) 5, which were sustained and increased to a large effect size by Wk 16 for all indications (Table). Similarly, clinically meaningful improvement in SF-12 MCS, CHQ-PF50 PhS, and CHQ-PF50 PsS scores was observed for all indications, except for PsS in HIDS/MKD and TRAPS pts.
Conclusion: Canakinumab showed rapid improvement by Wk 5 in pt-reported outcomes in adults and children with PFS, which was sustained through Wk 16. References: 1. User’s manual for the SF-12v2 Health Survey, 3rd ed.; 2012 2. Cohen J, et al. Statistical power analysis for the behavioural sciences, 2nd ed.; 1988 3. HealthActCHQ. The CHQ Scoring and Interpretation Manual (Boston, MA: HealthActCHQ, 2013) 4. Maruish, M.E. ed. User’s manual for the SF-12v2 Health Survey, 3rd ed. (Lincoln, RI: QualityMetric Inc., 2012)
To cite this abstract in AMA style:
Simon A, Shcherbina A, Anton J, Ben-Chetrit E, De Benedetti F, Frenkel J, Gattorno M, Hara R, Hashkes PJ, Hofer M, Hoffman HM, Koné-Paut I, Lachmann H, Martini A, Ozen S, Zeft A, Speziale A, Junge G, Gregson J. Effect of Canakinumab Treatment on Health-Related Quality of Life in Patients with Periodic Fever Syndromes [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/effect-of-canakinumab-treatment-on-health-related-quality-of-life-in-patients-with-periodic-fever-syndromes/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-canakinumab-treatment-on-health-related-quality-of-life-in-patients-with-periodic-fever-syndromes/