Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: While the use of biologics over the last decade has revolutionized treatment for some patients with axial SpA, only up to 40% reach remission in placebo-controlled trials. With absent biomarkers, we rely on a “trial and error” approach leading to unreliable response, with often unnecessary side effects, and disease progression. Both C-reactive protein (CRP) levels and bone marrow edema on magnetic resonance imaging (MRI) have been associated with response to TNFi in axSpA. It is, however, unclear if combining these findings could provide better insight than either factor alone. In contrast to biomarkers, MRI and CRP are both usually performed at baseline in axSpA patients. Therefore, our objective was to perform a systematic review and meta-analysis of data from 4 subgroups (MRI+/CRP+, MRI+/CRP-, MRI-/CRP+, MRI-/CRP-) from randomized controlled trials (RCTs).
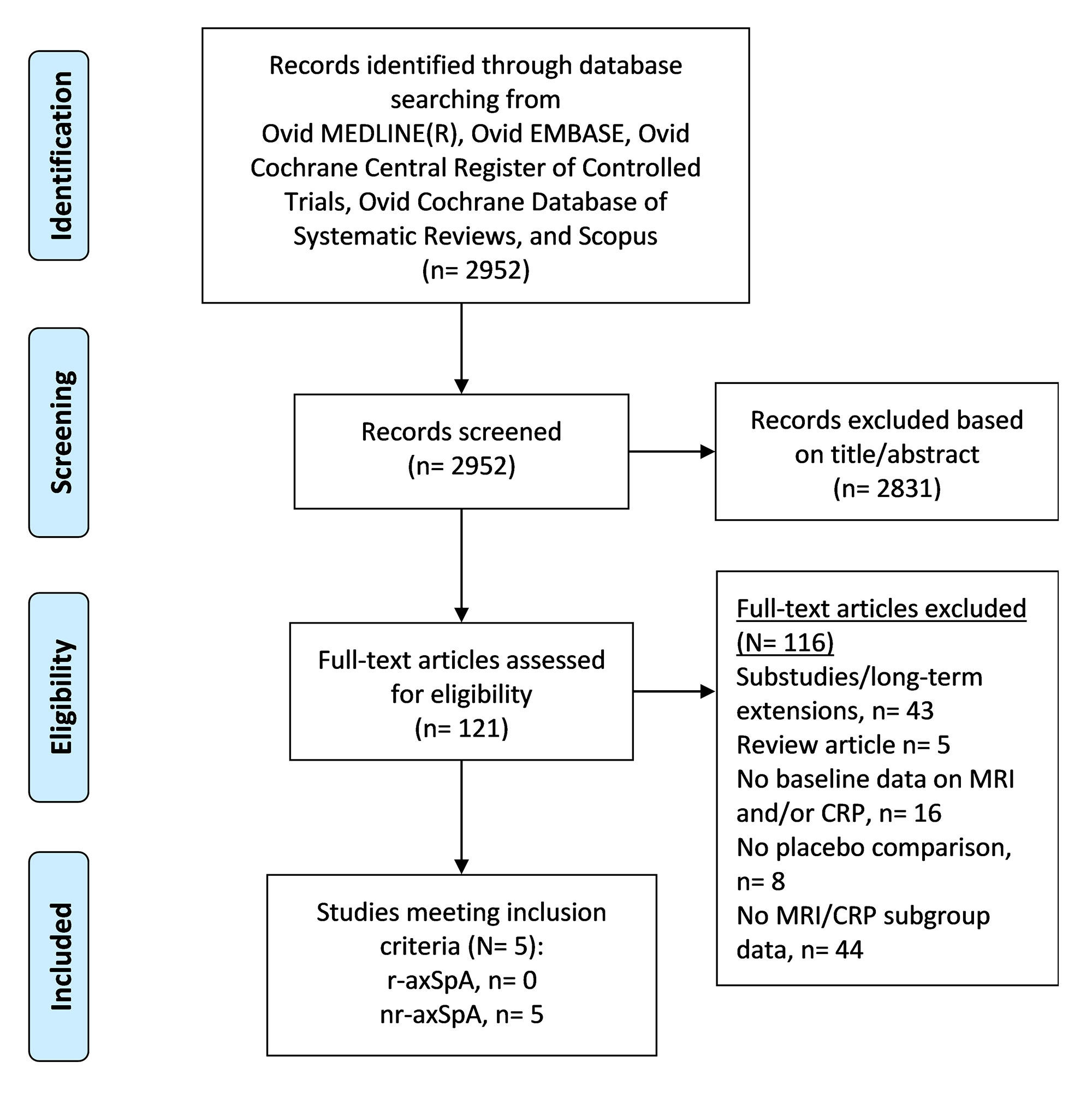
Methods: We performed a comprehensive search of databases in all languages (including conference abstracts) up to Jan 11, 2022. RCTs (phase II & III) with these criteria were included: 1) adults (≥18 years) with axSpA (r-axSpA and nr-axSpA), 2) disease activity assessed using a quantitative scoring method, 3) disease activity reported for a particular therapy compared to placebo, 4) disease activity reported by different MRI/CRP subgroups, 5) MRI and CRP performed at baseline for >50% of participants. Number needed to treat (NNT) was calculated as the inverse of the risk difference. Risk of bias in the included studies was assessed using the Revised Cochrane risk-of-bias tool (RoB 2.0) for randomized trials. Between-study heterogeneity was assessed using I2 statistics and sensitivity analyses were performed to explore heterogeneity. Study-specific disease activity scores (at 12 to 16 weeks) were pooled using a random-effects model (DerSimonian and Laird).
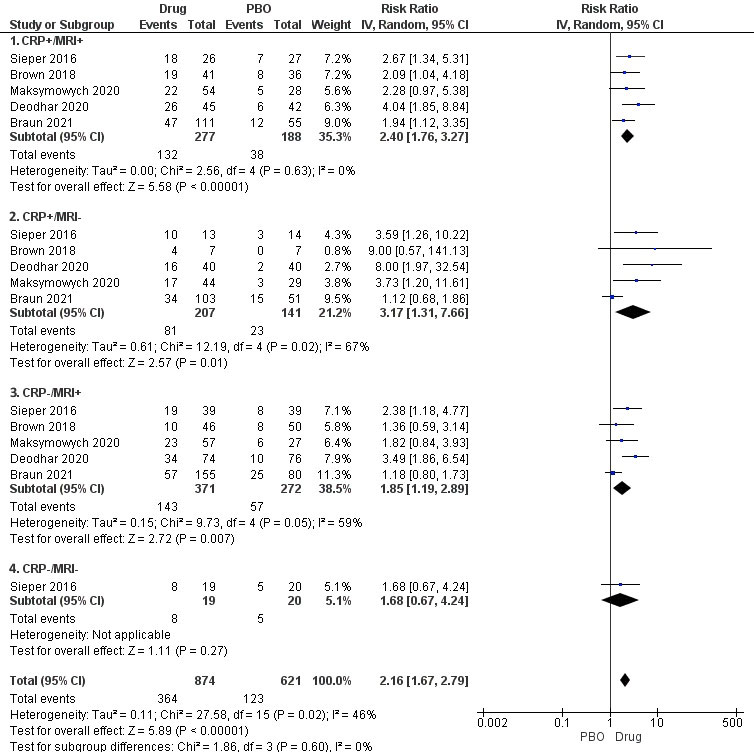
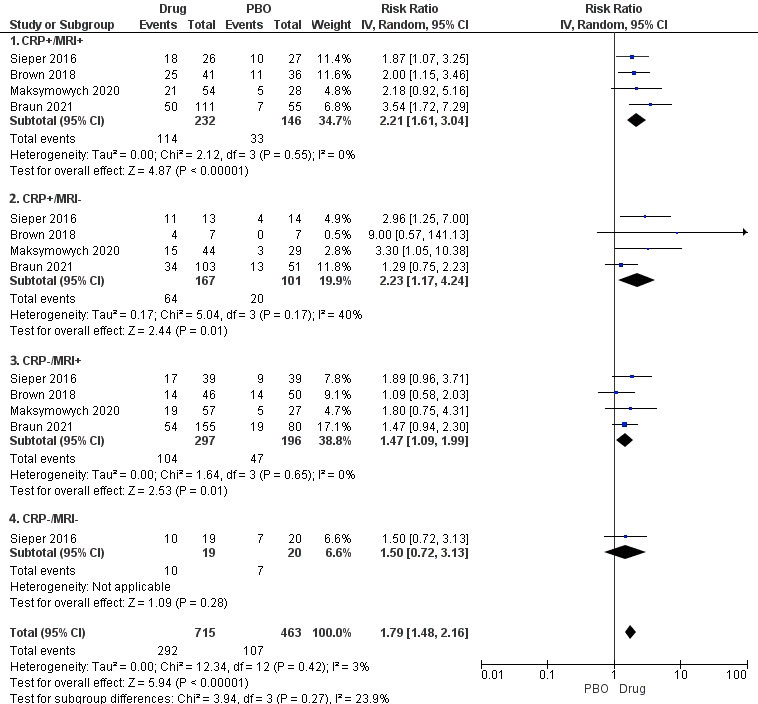
Results: Five studies (all nr-axSpA) were included- 3 with tumor necrosis factor inhibitors (TNFi, N= 729), 2 with interleukin-17 inhibitors (IL-17i, N=794). TNFi and IL-17i showed efficacy based on ASAS40 and BASDAI50 in all MRI/CRP subgroups except CRP-/MRI- subgroup, which had a single study with only 39 patients (Figure 1). There was no statistically significant difference between the four subgroups in terms of patients achieving ASAS40 (p=0.60, I2=0%) or BASDAI50 (p=0.27, I2-23.9%). NNT was 3 for the CRP+/MRI+ and CRP+/MRI- subgroups, and 6 for the CRP-/MRI+ and CRP-/MRI- subgroups. All studies had a low risk of bias. Between-study heterogeneity was low to moderate. Sensitivity analyses comparing TNFi or IL-17i vs. placebo similarly showed no difference between subgroups in terms of ASAS40 (TNFi, p=0.57, and IL-17i, p=0.28) and BASDAI50 (TNFi, p=0.37, and IL-17i, p=0.18).
Conclusion: In this systematic review, all subgroups showed efficacy based on ASAS40 or BASDAI50 except the CRP-/MRI- subgroup, which was underpowered and reported in a single study. There was no difference in outcomes between subgroups based on MRI and CRP positivity. However, numerically higher treatment responses were noted with a higher burden of inflammation in terms of CRP.
To cite this abstract in AMA style:
Karmacharya P, Gupta S, Shahukhal R, Khanal R, Murad H, Gensler L. Effect of Biologics in MRI/CRP Subgroups of Axial Spondyloarthritis: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effect-of-biologics-in-mri-crp-subgroups-of-axial-spondyloarthritis-a-systematic-review-and-meta-analysis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-biologics-in-mri-crp-subgroups-of-axial-spondyloarthritis-a-systematic-review-and-meta-analysis/