Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The risk for cardiovascular disease (CVD) is increased in patients (pts) with RA. The interplay between traditional CV risk factors and inflammatory burden may be responsible for this increased CVD risk. Therefore, CVD risk scores incorporating measures of inflammation such as the Reynolds risk score (RRS) may be more appropriate than the Framingham risk score or total cholesterol/HDL ratio to predict CVD risk in pts with rheumatoid arthritis (RA), though not yet validated for RA pts (Ridker, 2007, 2008). This study investigated the effects of biologics on lipids, CRP and CVD risk using RRS.
Methods: Pts with moderate disease activity (CDAI >10) initiating a biologic DMARD participated in a comparative effectiveness prospective study (CERTAIN) nested within CORRONA. Characteristics, including LDL, HDL, total cholesterol (TC) (desirable < 200) and triglycerides (TG), high sensitivity C-reactive protein (hsCRP) and RRS in pts initiating a TNF-α inhibitor (TNFi) or non‐TNFi (rituximab [RTX], abatacept [ABT] or tocilizumab [TCZ]), were measured at baseline and at 3 months. Linear, mixed effect regression models were fit to compare the effect of biologics on lipids, CRP and RRS outcomes after 3 months of therapy. In all models adjustment took place for the baseline levels of the outcome, biologically plausible covariates and other characteristics that were imbalanced among the four groups of medications.
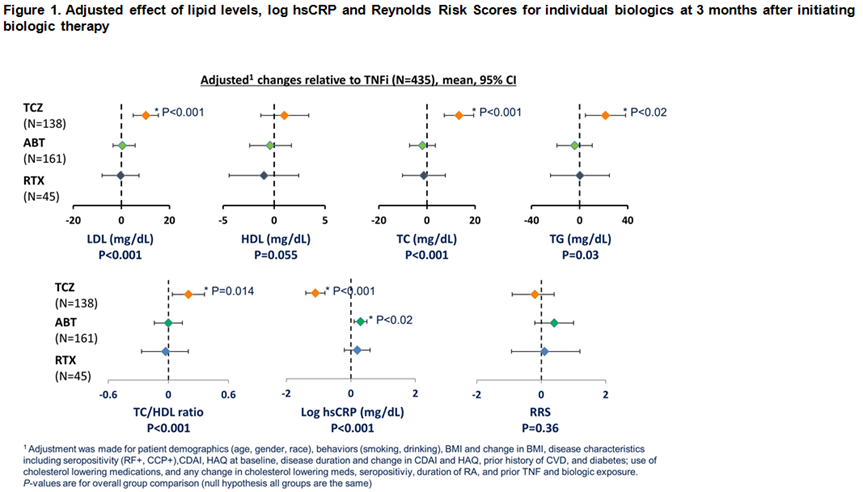
Results: 779 initiations of a biologic were analyzed: 435 (55.8%) TNFi, 45 (5.8%) RTX, 161 (20.7%) ABA and 138 (17.7%) TCZ. Overall baseline characteristics: 75.9% women, 86.3% Caucasian, 65.5% seropositive. Mean ± SD age was 55.9 ± 13.3 years, RA disease duration 8.8 ± 9.5 years; CDAI 28.6 ± 12.7. 35.9% of pts were biologic naïve and 24.6% received anti-hyperlipidemic therapy. History of prior CVD was present in 8.0% of pts; 7.8% had diabetes mellitus and 41% were obese (BMI > 30). The adjusted effect of biologic agents on lipid levels, CRP and RRS after 3 months of treatment are summarized in Figure 1. ABT and RTX had a similar effect on lipid levels compared to TNFi after 3 months while TCZ increased TC, LDL and TG levels compared with TNFi after 3 months. Significantly different changes in CRP were observed across different biologics at 3 months. For CVD risk, as measured by RRS, ABT, RTX and TCZ had a similar effect compared with TNFi.
Conclusion: Our study showed that there was a differential effect on lipid levels and CRP when compared amongst biologics with different mechanisms of action. However, these changes did not translate to an increased CVD risk as measured by RRS. In order to better understand CV risk in RA, long term outcome studies are needed to evaluate the interplay among lipids, inflammation and other CVD risk factors.
Ridker P, et al. JAMA 2007;297:611; Ridker P, et al. Circulation 2008;118;22
Disclosure:
D. A. Pappas,
CORRONA ,
3,
Novartis ,
5;
A. John,
Genentech ,
3;
J. R. Curtis,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, AbbVie,
2,
Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, BMS, Crescendo, AbbVie,
5;
G. W. Reed,
CORRONA ,
3;
J. D. Greenberg,
CORRONA ,
1,
Astra Zeneca, Corrona, Novartis, Pfizer,
5;
A. Shewade,
Genentech ,
3;
D. H. Solomon,
Amgen, Lilly, CORRONA,
2,
Pfizer, Novartis, Lilly, BMS,
9,
UpToDate,
7;
J. M. Kremer,
CORRONA ,
1,
CORRONA ,
3;
T. Sommers,
CORRONA ,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-biologic-agents-on-lipids-and-cardiovascular-risk-in-rheumatoid-arthritis-patients/