Session Information
Date: Monday, November 9, 2015
Title: Miscellaneous Rheumatic and Inflammatory Diseases Oral Session II
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: To compare the
effects of adalimumab and placebo on the National Eye Institute Visual
Functioning Questionnaire 25 (VFQ-25) in subjects requiring high dose
corticosteroids for active non-infectious intermediate-, posterior-, or
pan-uveitis.
Methods:
The
VISUAL-1 clinical trial (NCT01148225) was a phase 3, randomized
placebo-controlled study. It investigated the efficacy and safety of adalimumab
(80 mg loading dose followed by 40 mg every other week) as maintenance therapy
in subjects with active non-infectious intermediate-, posterior- or pan-uveitis.
The VFQ-25 is a validated measure for assessing the impact of visual impairment
from the patient’s perspective. The VFQ-25 total score is calculated as the
mean of 11 vision-related domains and scores range from 0 (worst vision functioning)
to 100 (best vision functioning). The VFQ-25 was administered at every
scheduled study visit of the 80 Week trial. As a ranked secondary outcome, the
change in VFQ-25 from best state achieved prior to Week 6 to the final/early
termination visit was compared between adalimumab and placebo using ANOVA. To
investigate the temporal effects of adalimumab and placebo on VFQ-25 in a
robust manner, a longitudinal GEE model (which incorporated all VFQ-25 measurements)
was estimated.
Results:
The
VISUAL-1 clinical trial enrolled a total of 217 subjects (110 adalimumab, 107
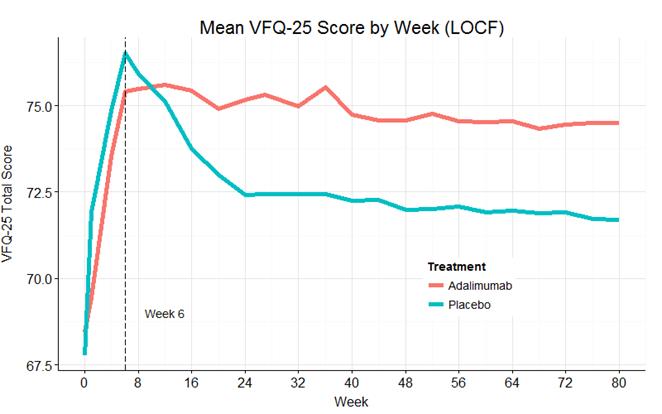
placebo). The mean VFQ-25 total scores for adalimumab and placebo are similar through
the tapering period but subsequently diverge and maintain separation through
week 80 (Figure 1). The average change in VFQ-25 total score from best
state achieved prior to Week 6 to the final / early termination visit was -5.50
for placebo and -1.30 for adalimumab. This corresponds to a statistically
significant and clinically meaningful1 increase of 4.20 (95%
confidence interval [CI]: 1.02 – 7.38; P = 0.010) associated with adalimumab
relative to placebo. The longitudinal model estimated a statistically
significant treatment effect of adalimumab of 3.07 (95% CI: 2.09 – 4.06; P<0.001).
Conclusion:
Treatment
with adalimumab is associated with statistically significant improvements in
visual functioning for subjects with active non-infectious non-anterior
uveitis.
Reference: [1] Naik RK et al.
Qual Life Res. 2013;22:2801–08.
Figure
1: Mean VFQ-25 Over Time
To cite this abstract in AMA style:
Sheppard J, Joshi AD, Mittal M, Betts K, Tari S, Bao Y, Dick AD. Effect of Adalimumab on Visual Functioning (VFQ-25) in Visual-1 Trial Patients with Non-Anterior Non-Infectious Uveitis [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/effect-of-adalimumab-on-visual-functioning-vfq-25-in-visual-1-trial-patients-with-non-anterior-non-infectious-uveitis/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-adalimumab-on-visual-functioning-vfq-25-in-visual-1-trial-patients-with-non-anterior-non-infectious-uveitis/