Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Anti-citrullinated protein antibody (ACPA) is a marker for early, erosive RA.1 In the Abatacept (ABA) versus adaliMumab (ADA) comParison in bioLogic-naïvE RA subjects with background MTX (AMPLE; NCT00929864) trial, higher versus lower baseline (BL) ACPA IgG levels were associated with improved clinical outcomes, and in patients (pts) with high ACPA IgG levels, ABA was associated with improved outcomes compared with ADA.2 The persistent presence of IgM is more indicative of continuous immune activation than IgG.1 In the Assessing Very Early Rheumatoid arthritis Treatment (AVERT) trial, ABA + MTX had greater clinical efficacy in pts who were ACPA IgM positive (+) compared with ACPA IgM negative (–) at BL, and in seroconverters versus non-seroconverters.3 This post hoc analysis of the AMPLE trial compared the treatment differences between ABA and ADA over 2 years, in pts who were ACPA IgM+ at BL by Day (D) 365 seroconversion status.
Methods: The AMPLE trial (NCT00929864) has been published.4 Samples, including those from ACPA+ pts were analyzed by ELISA to determine ACPA IgM serostatus. Outcomes over 2 years of treatment were analyzed by treatment group, based on BL ACPA IgM serostatus, and seroconversion to ACPA IgM– status, at D365. Analysis of covariance models were used to assess the adjusted mean changes, with treatment as factor and BL values and screening DAS28 (CRP) randomization stratification as covariates.
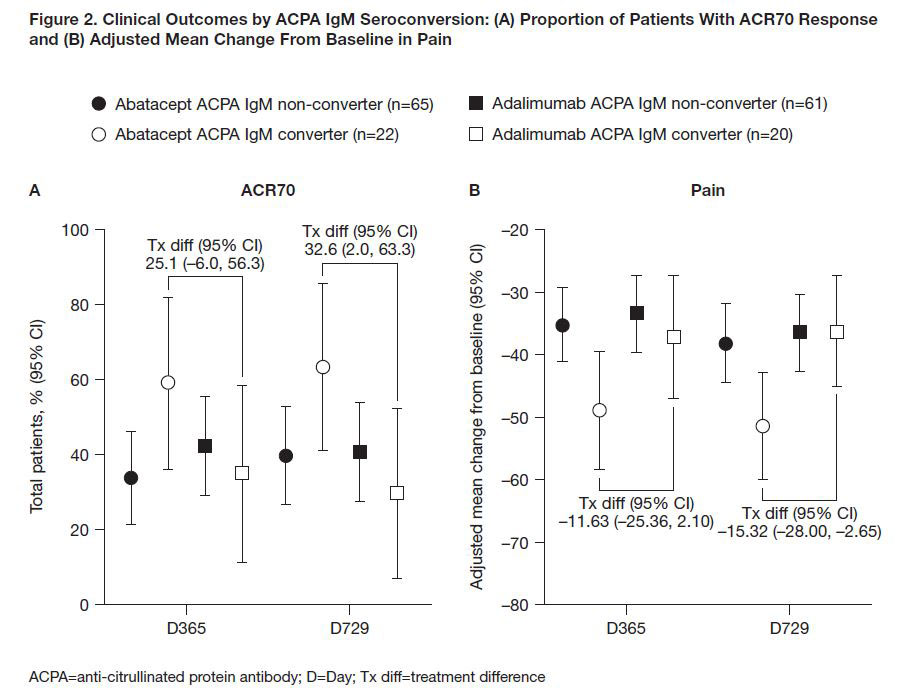
Results: Of 646 pts with RA (1987 ACR criteria5) in the AMPLE study, BL ACPA IgM status was available for 510 pts: 308 ACPA IgM– and 202 ACPA IgM+. BL characteristics were comparable between ACPA IgM+ and ACPA IgM– groups, and among all ACPA IgM+ pts across treatment arms between seroconverters at D365 versus non-converters. In pts who were ACPA IgM+ at BL, ABA was associated with a numerically higher ACR70 response rate (Figure 1A) and greater reduction in pain (Figure 1B) than ADA at D365 and D729. The trend in treatment difference for ACR70 and pain was consistent over time (data not shown). By D365, a similar proportion of pts from each treatment group seroconverted from ACPA IgM+ to ACPA IgM– status (Table 1). In pts who seroconverted from ACPA IgM+ at BL to ACPA IgM– at D365, ABA was associated with a greater numerical improvement in ACR70 response (Figure 2A) and reduction in pain (Figure 2B) than ADA at D365 and D729.
Conclusion: ACPA IgM seropositive status at BL predicted numerically better clinical outcomes after 365 and 729 days of treatment in abatacept-treated pts compared with ADA-treated pts. Among the subset of pts who seroconverted to ACPA IgM– status, abatacept treatment was associated with numerically better clinical responses. ACPA IgM+ status may help identify a subset of pts with active RA who respond better to abatacept treatment.
References:
- Verpoort KN, et al. Arthritis Rheum 2006;54:3799–808.
- Sokolove J, et al. Ann Rheum Dis 2016;75:709–14.
- Huizinga TWJ, et al. Ann Rheum Dis 2015;74:234–5 (THU0114).
- Schiff M, et al. Ann Rheum Dis 2014;73:86–94.
- Arnett FC, et al. Arthritis Rheum 1988;31:315–24.
Professional medical writing: Rachel Rankin, Caudex, funded by Bristol-Myers Squibb.
To cite this abstract in AMA style:
Huizinga T, Toes R, Weinblatt M, Schiff M, Fleischmann R, Elbez Y, Connolly S, Maldonado M, Gao S. Effect of ACPA IgM Serostatus on Efficacy Outcomes Following Treatment with Abatacept or Adalimumab: A Post Hoc Analysis of a Phase III Head-to-Head Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effect-of-acpa-igm-serostatus-on-efficacy-outcomes-following-treatment-with-abatacept-or-adalimumab-a-post-hoc-analysis-of-a-phase-iii-head-to-head-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-acpa-igm-serostatus-on-efficacy-outcomes-following-treatment-with-abatacept-or-adalimumab-a-post-hoc-analysis-of-a-phase-iii-head-to-head-trial/