Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Abatacept (ABT) is a new class of biologic agents for the treatment of rheumatoid arthritis (RA) that inhibits T cell activation by binding to CD80/86. It has been reported that ABT was less effective in the patients with previous biological DMARDs and concomitant MTX had only little enhancing effect on ABT efficacy. Tacrolimus (TAC) was approved in Japan for the treatment of RA (oral dosage of ≤ 3 mg/day). It down-regulates the synthesis of inflammatory cytokines activated by T cells mainly via inhibition of a calcineurin. In this study, we investigated whether concomitant TAC had enhancing effect on ABT efficacy in the patients with previous biological DMARDs, using data from a Japanese multicenter registry system (TBCR).
Methods
The present study included all patients who had previous biological DMARDs and were initiated intravenous ABT and prospectively observed for longer than 52 weeks at TBCR-affiliated institutes (n = 121). Demographic data and the following parameters of disease activity were collected; tender joint count (TJC) and swollen joint count (SJC), patient global assessment (VAS), ESR, and serum CRP at baseline, 4, 12, 24, and 52 weeks. We compared these clinical data between the patients treated without concomitant MTX or TAC (ABT-mono, n=42), those treated with concomitant MTX (ABT-MTX, n=56), and those treated with concomitant TAC (ABT-TAC, n=18). The last observation carried forward (LOCF) method was used in each analysis.
Results
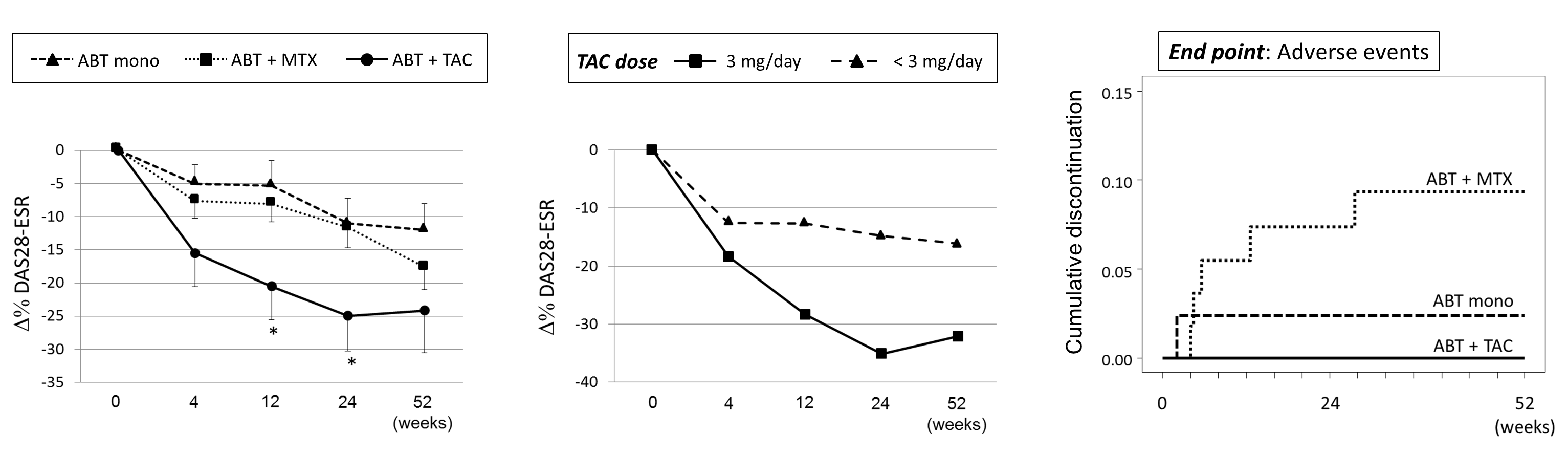
In the baseline characteristic data, the ABT-mono group had higher pulmonary comorbidity rate (23.8%, p = 0.030) compared to the ABT-MTX (5.4%) and ABT-TAC (16.7%) group while no other clinical parameters showed significant difference including all disease activity indices such as DAS28-ESR (5.10, 5.30, and 5.30, p = 0.949). The ABT-TAC group demonstrated significantly lower Ģ% of DAS28 score compared to the ABT-mono group while no difference between the ABT-mono and ABT-MTX group (Left panel). The patients taking 3 mg/day dosage of TAC demonstrated apparently lower Ģ% of DAS28 score compared to those taking < 3 mg/day (Middle panel). Kaplan-Meier analysis demonstrated that the ABT-MTX group showed significantly higher discontinuation rate due to adverse events compared to the ABT-mono group while no difference between the ABT-TAC and ABT-mono group (Right panel).
Conclusion
We clearly demonstrated that concomitant TAC treatment had dose-dependent enhancing effect on the ABT efficacy, while there seemed to be little combinational effect of ABT and MTX. Since both ABT and TAC are the agents targeting T cells, there is concern regarding safety issue when used in combination. However, in our results, the ABT-TAC group did not show increased discontinuation due to adverse events. We would suggest that combination of ABT and TAC treatment should be helpful when we treat the patients with previous biological DMARDs history.
Disclosure:
N. Takahashi,
Abbott Japan Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Co. Ltd, Chugai Pharmaceutical Co. Ltd., and Bristol-Myers Squibb Co. Ltd. ,
8;
T. Kojima,
Takeda Pharma Corporation, Janssen Pharmaceutical, and Astellas Pharma Corporation.,
2,
Mitsubishi Tanabe Pharma Corporation, Takeda Pharma Corporation, Eisai Pharma Corporation, Abbvie, Bristol-Myers Squibb、Pfizer and Chugai Pharma Corporation,
8;
Y. Hirano,
Abbott Immunology Pharmaceuticals,
8,
Mitsbishi-Tanabe Pharma,
8,
Pfizer Inc,
8,
Eisai,
8,
Chugai,
8,
Bristol-Myers Squibb,
8,
Astellas Pharma,
8;
Y. Kanayama,
Astellas Pharma,
8,
Eisai,
8,
Mitsubishi Tanabe Pharma Corporation,
8,
AbbVie Inc,
8,
Chugai,
8;
K. Funahashi,
Abbott Japan Co. Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Pfizer Co. Ltd, Chugai Pharmaceutical Co. Ltd., and Bristol-Myers Squibb Co. Ltd. ,
8;
N. Ishiguro,
AbbVie, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi Tanabe, Pfizer and Takeda.,
5,
AbbVie, Chugai, Daiichi-Sankyo, Eisai, Mitsubishi Tanabe, Pfizer and Takeda.,
8.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-and-safety-of-concomitant-methotrexate-and-tacrolimus-on-clinical-response-of-abatacept-in-rheumatoid-arthritis-patients-with-prior-use-of-biological-dmards/