Session Information
Date: Sunday, November 7, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster II: Manifestations (0855–0896)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Little is known about the economic burden of NP lupus. We estimated annual and cumulative direct and indirect costs (DC, IC) associated with NP events attributed to SLE and non-SLE causes using multistate modelling in a multicentre, multi-ethnic inception cohort.
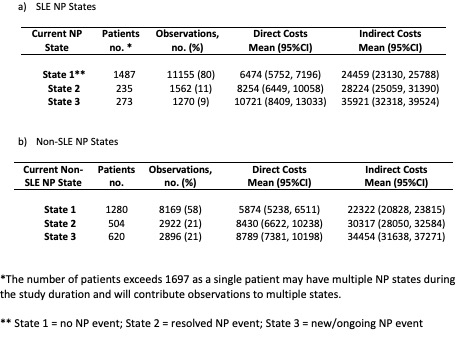
Methods: Patients fulfilling revised ACR classification criteria for SLE from 31 centres in 11 countries were enrolled within 15 months of diagnosis. NP events were documented annually using ACR NP case definitions and attributed to either SLE or non-SLE causes. At each assessment and for both SLE and non-SLE events, patients were stratified into 1 of 3 NP states (no, resolved, or new/ongoing NP event). The change in NP status characterized by transition rates between states was analyzed over time, using multistate modelling for SLE attributed NP events and non-SLE attributed NP events (Hanly. A&R 2021; doi: 10.1002/art.41876) (Fig 1).
At each assessment, annual DC and IC were based on health resource use and lost work-force/non-work-force productivity over the preceding year. Resource use was costed using 2021 Canadian prices and lost productivity using Statistics Canada age-and-sex specific wages. Costs associated with each SLE and non-SLE NP state were calculated by averaging all observations in each NP state. Multiple regressions including age at diagnosis, sex, race/ethnicity, disease duration, geographic region, and smoking adjusted for possible confounding of these variables on the association of annual DC and IC and NP state. 5 and 10-year cumulative costs for each NP state were predicted by multiplying adjusted annual costs associated with each state by the expected duration in each state, forecasted using the multistate model.
Results: 1697 patients (89% female, 51% non-Caucasian race/ethnicity, mean age at enrolment 35.1 years) were followed for a mean of 8.8 years. 1971 NP events occurred in 956 patients, 32% attributed to SLE. Across 13,987 assessments, the majority of observations were provided by patients with no NP event (Table 1.) For the SLE NP events, annual DC were higher in those with a new/ongoing vs no event ($10809 vs $6715) (Table 2). Although 5 and 10-yr cumulative DC trended higher in the new/ongoing vs no event, the differences were not significant. However, annual and 5-yr IC were higher in the new/ongoing vs no event and new/ongoing vs resolved event. (5-yr: new/ongoing vs no: $172674 vs $ $136970). For the non-SLE NP events, although all DC trended higher in the new/ongoing vs no event, the differences were not significant. However, annual IC were higher in the new/ongoing vs no event, new/ongoing vs resolved event, and resolved vs no event and 5 and 10-yr IC were higher in the new/ongoing vs no event (10-yr: new/ongoing vs no: $342434 vs $279874). For all NP states, IC exceeded DC between 2.8 and 4-fold.
Conclusion: IC are approximately 1.3-fold higher in patients with new/ongoing vs no NP events, attributed to either SLE or non-SLE. While DC trended higher in those with new/ongoing events, they did not differ significantly. Impaired productivity associated with both ongoing and resolved NP lupus is substantial and underscores the previously documented reduced quality of life in NP lupus.
To cite this abstract in AMA style:
Clarke A, Hanly J, St.Pierre Y, Gordon C, Bae S, Romero-Diaz J, Sanchez-Guerrero J, Bernatsky S, Wallace D, Isenberg D, Rahman A, Merrill J, Fortin P, Gladman D, Urowitz M, Bruce I, Petri M, Ginzler E, Dooley M, Ramsey-Goldman R, Manzi S, Jnsen A, Alarcn G, van Vollenhoven R, Aranow C, Mackay M, Ruiz-Irastorza G, Lim S, Inanc M, Kalunian K, Jacobsen S, Peschken C, Kamen D, Askanase A, Farewell V. Economic Evaluation of Neuropsychiatric (NP) Lupus in an International Inception Cohort Using a Multistate Model Approach [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/economic-evaluation-of-neuropsychiatric-np-lupus-in-an-international-inception-cohort-using-a-multistate-model-approach/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/economic-evaluation-of-neuropsychiatric-np-lupus-in-an-international-inception-cohort-using-a-multistate-model-approach/