Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Clinical studies have shown that a large proportion of psoriatic arthritis (PsA) patients treated with apremilast failed to achieve improved physical function or experienced severe gastrointestinal (GI) symptoms. This study used administrative claims data to evaluate the proportion of biologic treatment-naïve patients experiencing early treatment failure with apremilast, identify characteristics associated with early treatment failure with apremilast, and assess subsequent treatment use after apremilast discontinuation in the real-world setting.
Methods: Adult biologic-naïve patients with PsA and apremilast use during Q1 2013 to Q3 2016 were selected from the IBM MarketScan Research Database (Copyright © 2017 Truven Health Analytics LLC, All Rights Reserved). The index date was defined as the first claim for apremilast after PsA diagnosis. Patients were required to have continuous enrollment during the 6 months before (baseline period) and the 6 months after the index date (study period). Early treatment failure was defined as the occurrence of treatment discontinuation, GI-related healthcare encounters, treatment switching/addition, or dose escalation/reduction within the 6-month study period. Time to treatment failure was described using the Kaplan-Meier method. A logistic regression model was used to assess characteristics associated with early treatment failure with apremilast. Subsequent treatment use was assessed through the end of continuous enrollment among patients who discontinued apremilast within 6 months.
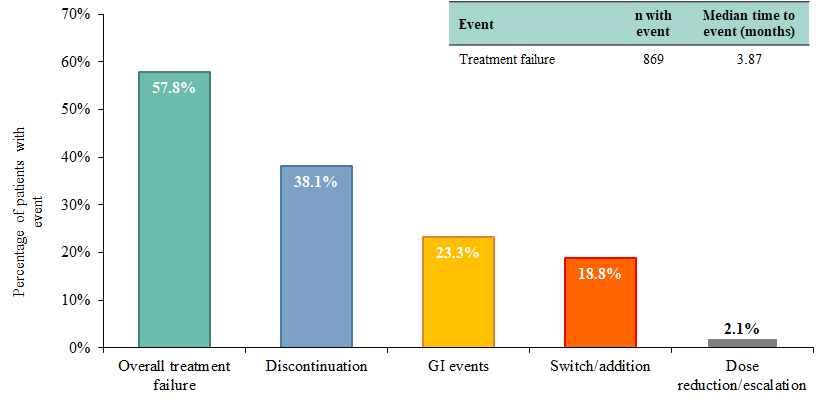
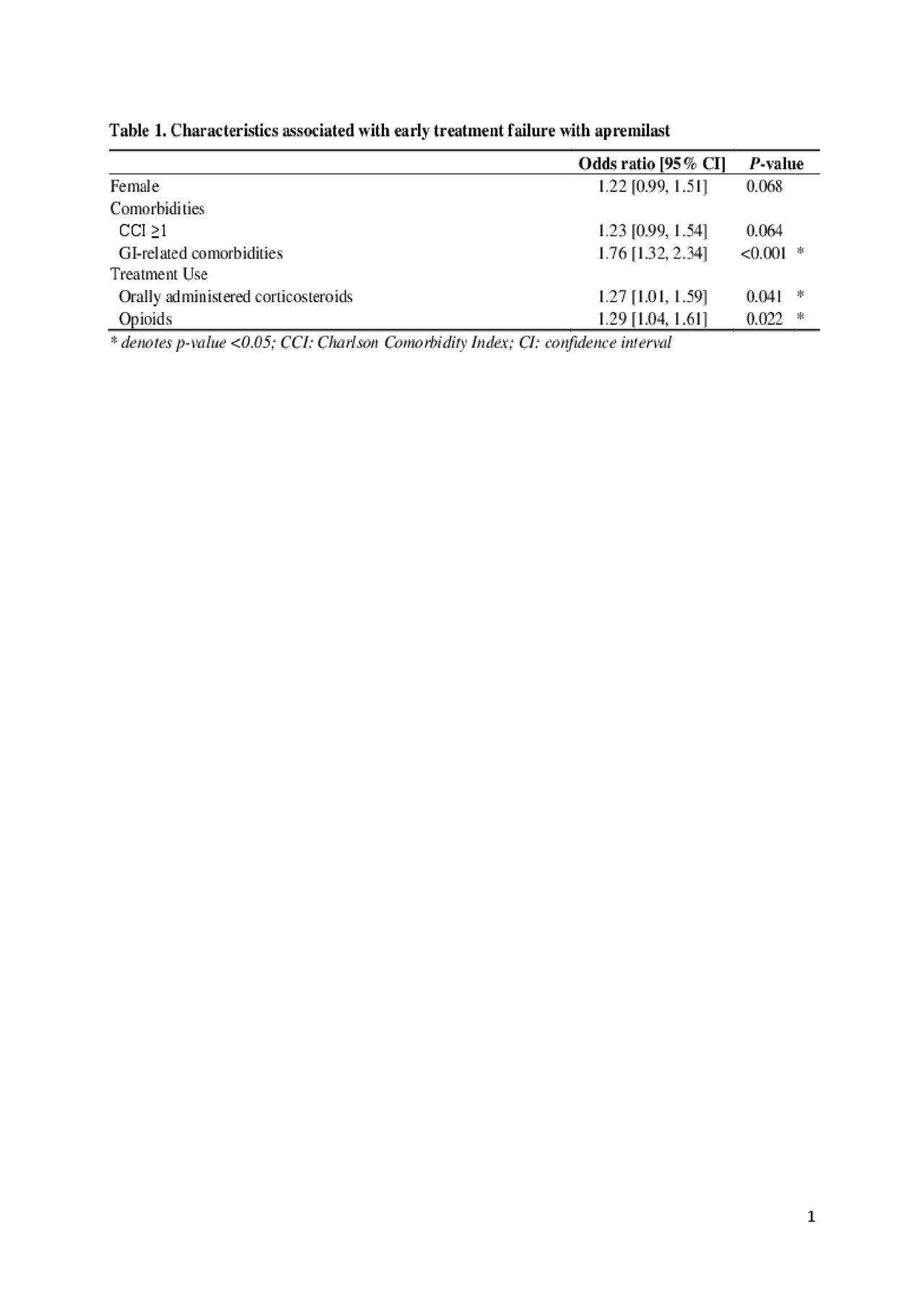
Results: A total of 1,503 biologic-naïve patients with PsA who were treated with apremilast were included, 869 (57.8%) with and 634 (42.2%) without early treatment failure. Median time to apremilast failure was 3.9 months and treatment discontinuation was the most common treatment failure event (Figure 1). Clinically relevant baseline characteristics that were significantly different between patients with and without early apremilast failure were included in the model (Table 1). Presence of GI-related comorbidities (odds ratio [OR]: 1.76, p< 0.001), orally administered corticosteroid use (OR: 1.27, p=0.04), and opioid use (OR: 1.29, p=0.02) were significantly associated with a higher risk of early treatment failure with apremilast. Among the 572 patients (38.1%) who discontinued apremilast within 6 months, 46.5% did not have subsequent treatment use; 20.3% subsequently initiated adalimumab, 15.7% subsequently initiated etanercept, 9.2% subsequently initiated secukinumab, and 9.2% subsequently initiated ustekinumab.
Conclusion: This retrospective claims study showed that approximately 60% of biologic-naïve PsA patients treated with apremilast experienced treatment failure within 6 months. Presence of GI-related comorbidities, use of orally administered corticosteroids, and use of opioids before apremilast initiation were associated with an increased risk of early treatment failure. In addition, approximately 54% of patients initiated a subsequent treatment following apremilast discontinuation. The results of this real-world study are consistent with findings from clinical trials.
To cite this abstract in AMA style:
Betts K, Patel P, Song J, Zhao J, Wang Y, Griffith J, Wu E. Early Treatment Failure with Apremilast Among Biologic-naïve Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/early-treatment-failure-with-apremilast-among-biologic-naive-patients-with-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-treatment-failure-with-apremilast-among-biologic-naive-patients-with-psoriatic-arthritis/