Session Information
Date: Tuesday, November 14, 2023
Title: (2227–2256) Spondyloarthritis Including Psoriatic Arthritis – Treatment: SpA Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Guselkumab (GUS), a fully human IL-23p19 subunit inhibitor, was shown to reduce mean changes in radiographic progression vs placebo (PBO) by week (W)24 and to be associated with low rates of radiographic progression through W100 among GUS-treated patients (pts) with PsA, irrespective of dosing regimen (every [Q] 4W or Q8W). Furthermore, earlier clinical response predicted improved long-term radiographic outcome in GUS-treated pts with active PsA. The recently developed 3 Visual Analogue Scale (3VAS) and 4VAS scores are the first short multidimensional composite measures specifically for use in PsA routine clinical care. Here we determined whether early improvement in 3VAS/4VAS predicts radiographic change through W100.
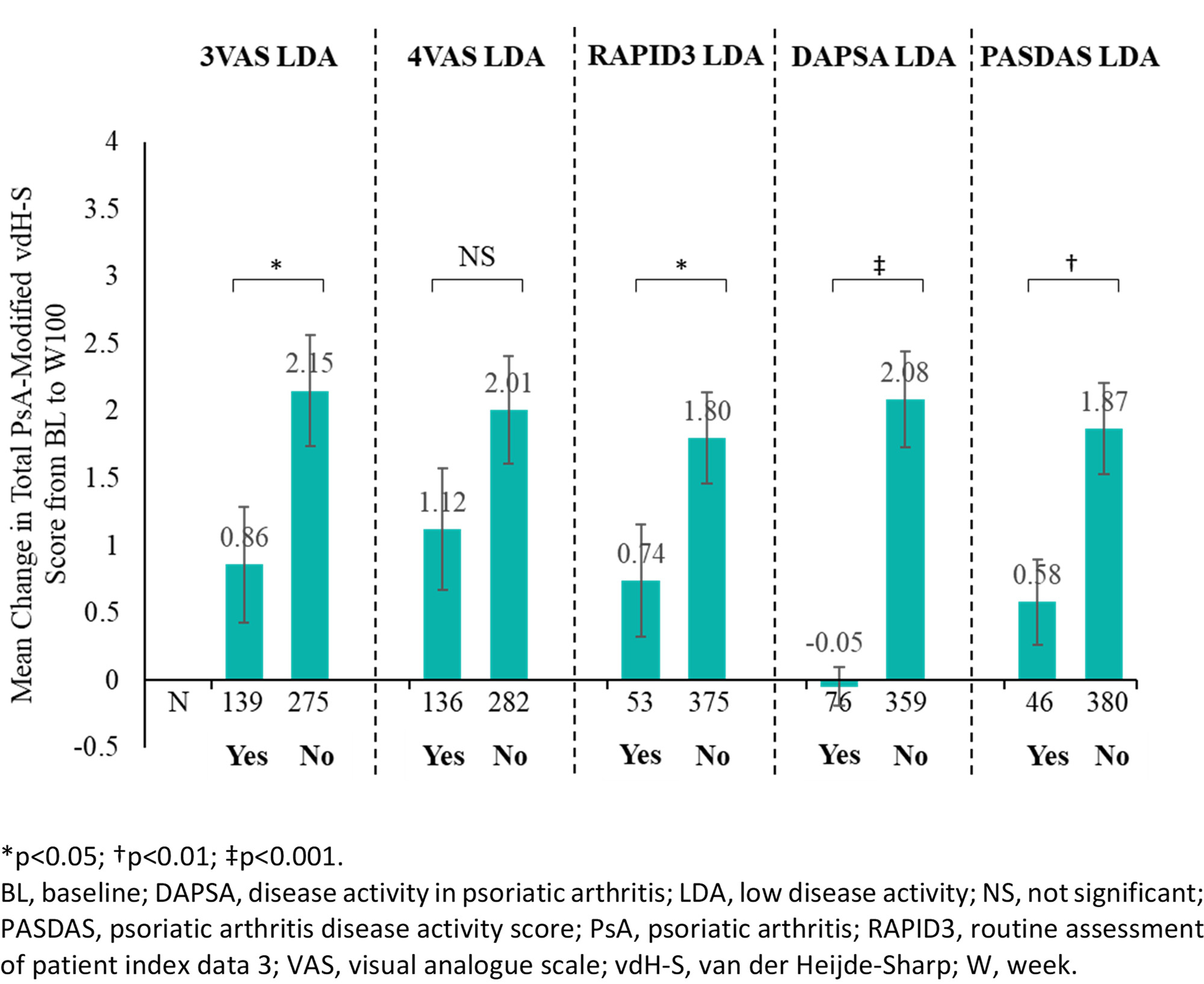
Methods: DISCOVER-2 included bio-naive pts with active PsA (≥5 swollen and ≥5 tender joint counts [SJC/TJC]; CRP ≥0.6 mg/dL) randomized (1:1:1) to GUS 100 mg Q4W; GUS 100 mg at W0, W4, then Q8W; or PBO with crossover to GUS 100 mg Q4W at W24. In the current analysis, only pts randomized to GUS (Q4W and Q8W) were included (N=493). Response at W8 was defined as achievement of low disease activity (LDA) in 3VAS (≤3.4), 4VAS (≤3.5), routine assessment of pt index data 3 (RAPID3; ≤6), Disease Activity in PsA (DAPSA; ≤14), and PsA Disease Activity Score (PASDAS; ≤3.2). Association of W8 response with change from baseline (BL) to W100 in total PsA-modified van der Heijde-Sharp (vdH-S) score was assessed with the independent samples t-test and generalized linear models adjusting for known BL determinants of radiographic progression (vdH-S score, age, gender, and CRP). Pairwise correlations and agreement in LDA classification between the endpoints were assessed with Pearson’s correlation coefficient and the kappa statistic, respectively.
Results: Among GUS-treated pts not meeting the respective endpoints at BL, 32.9%, 31.6%, 12.4%, 17.8%, and 10.8% achieved LDA in 3VAS, 4VAS, RAPID3, DAPSA, and PASDAS, respectively, at W8. LDA achievement in 3VAS (0.86 vs 2.15, p=0.03), RAPID3 LDA (0.74 vs 1.80, p=0.049), DAPSA LDA (-0.05 vs 2.08, p< 0.001), and PASDAS LDA (0.58 vs 1.87, p=0.006) at W8 were associated with significantly less radiographic progression through W100 (Figure). For 4VAS, achievement of remission (≤2.1; 0.71 vs 1.84, p=0.045), but not LDA (1.12 vs 2.01, p=0.142), was also associated with improved radiographic outcome. In multivariate analyses, improved response to GUS treatment at W8 in all endpoints assessed was associated with numerically less radiographic progression through W100. At W8, 3VAS and 4VAS showed strong correlations with RAPID3 (r3VAS=0.787; r4VAS=0.877) and PASDAS (r3VAS=0.795; r4VAS=0.790) and moderate correlations with DAPSA (r3VAS=0.466; r4VAS=0.524), whereas fair to moderate agreement (kappa range: 0.325-0.545) in LDA classification was noted.
Conclusion: Approximately one-third of GUS-treated pts achieved early response (W8 LDA) in 3VAS/4VAS, which was associated with reduced rates of radiographic change, as was early response in the other outcomes assessed. These results suggest that, in addition to their usefulness in assessing disease activity in routine clinical care, 3VAS and 4VAS, the former being more sensitive, may predict long-term radiographic changes.
To cite this abstract in AMA style:
Tillett W, Coates L, vis m, Soriano E, Merola J, Zimmermann M, Shawi M, Sharaf M, Nash P, Helliwell P. Early Improvement in 3 Visual Analogue Scale (3VAS)/4VAS Predicts Reduced Rates of Radiographic Change in Bio-naive Active Psoriatic Arthritis Patients Receiving Guselkumab Treatment [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/early-improvement-in-3-visual-analogue-scale-3vas-4vas-predicts-reduced-rates-of-radiographic-change-in-bio-naive-active-psoriatic-arthritis-patients-receiving-guselkumab-treatment/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-improvement-in-3-visual-analogue-scale-3vas-4vas-predicts-reduced-rates-of-radiographic-change-in-bio-naive-active-psoriatic-arthritis-patients-receiving-guselkumab-treatment/