Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: ANCA-associated vasculitis (AAV) is a small-to-medium vessel vasculitis associated with substantial morbidity and mortality, in part due to glucocorticoid exposure. Avacopan, an oral C5a inhibitor, is non-inferior to a standard glucocorticoid regimen, as part of usual remission induction treatment for AAV. Avacopan is increasingly used but little is known about real-world experience with this drug.
Methods: We identified all prescriptions for avacopan in a large integrated healthcare system in the US that includes 12 hospitals, including their primary care practices, community health centers, specialty clinics, and inpatient settings. The study period was from the date of FDA approval of avacopan (October 8, 2021) through March 30, 2023. Patients were followed through May 15, 2023. Details extracted from the medical record included: date of initiation, reason for non-initiation (if applicable), ANCA type, BVAS/GPA, glucocorticoid use, duration of avacopan use, flares, and concomitant immunosuppression. We used the Kaplan-Meier method to estimate the time to treatment without glucocorticoids after avacopan initiation.
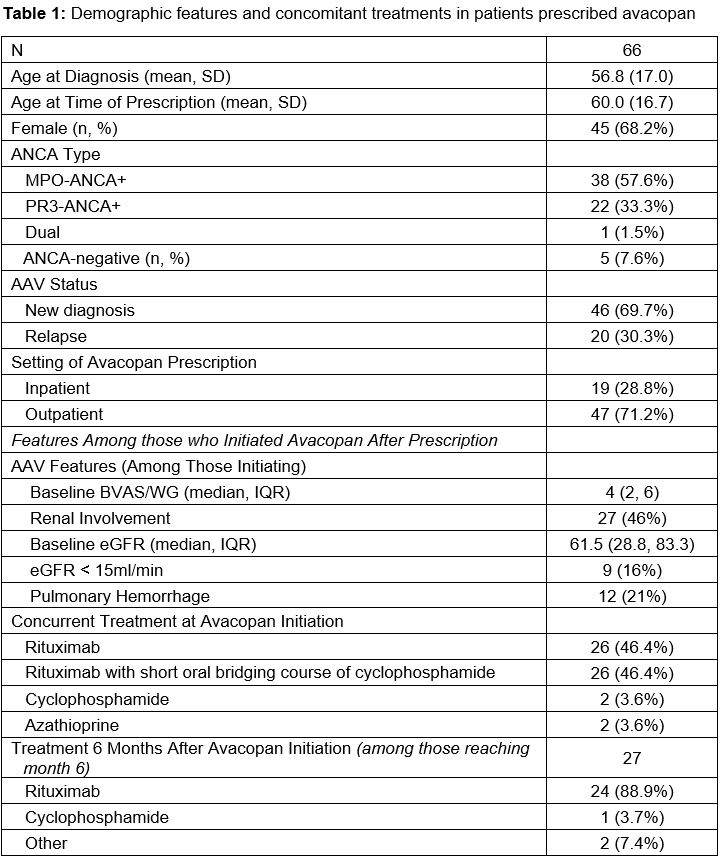
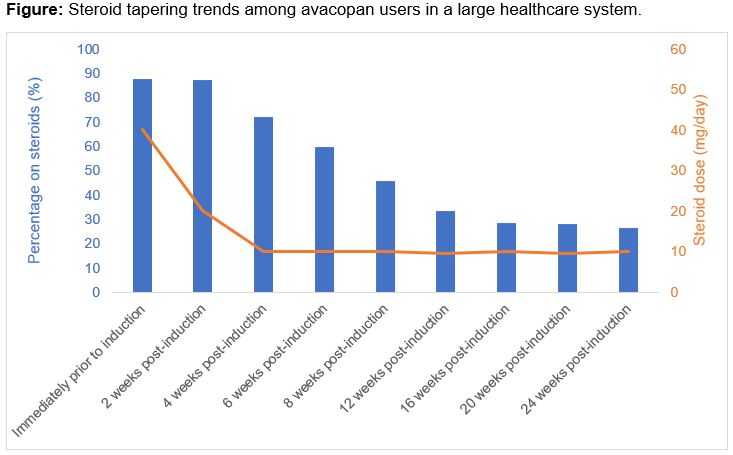
Results: Avacopan has been prescribed to 66 patients (Table 1). The mean age was 66 years; the majority were female (68.2%), MPO-ANCA+ (57.6%), and newly diagnosed (69.7%). Most initial prescriptions were in the outpatient setting (71.5%). Of the 66 patients, 56 (84.9%) began avacopan; 10 (15.1%) never initiated avacopan because of insurance-related barriers (n=5), intubation (n=2), and patient preference (n=1). The median time from prescription to initiation was 14.5 days. The most common induction regimens were rituximab (46.4%) or rituximab with a short oral course of cyclophosphamide (46.4%). Of the 56 patients who began avacopan, 49 (87.5%) were also treated with steroids. The median time to discontinuing steroid was 56 (28-168) days (Figure). At 8 weeks, 45.7% remained on prednisone (median dose 10mg/d). At 24 weeks, 26.5% were on prednisone (median dose 10mg/d). The median time on avacopan was 159 days (total exposure of 9,184 days). Avacopan was discontinued by 19 (33.9%), most often because of completion of the intended course (n=10; at 6 months in n=8). Avacopan was discontinued by 5 (8.9%, 0.05/100 person days) because of an adverse event (e.g., transaminitis, paresthesia, pruritis). Ten (18%) patients had a disease flare; 6 were minor and the median time to flare was 77 days (Table 2). Five patients were on steroids at the time of flare and increased their dose; the other five started prednisone. One patient who initiated avacopan died during their initial presentation because of hypoxemic respiratory failure attributed to infection.
Conclusion: This is among the first reports of real-world experience with avacopan. Avacopan users quickly tapered prednisone doses but low-to-moderate doses of prednisone were commonly used for prolonged periods. Nearly one-in-five patients experienced a flare. We identified insurance-related barriers to access. Studies are needed to determine the optimal use of steroids with avacopan and factors associated with relapse.
To cite this abstract in AMA style:
Srivatsan S, Williams Z, Cook C, Fu X, Patel N, Wallace Z. Early Experience with Avacopan for ANCA-Associated Vasculitis in a Large Integrated Healthcare System [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/early-experience-with-avacopan-for-anca-associated-vasculitis-in-a-large-integrated-healthcare-system/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-experience-with-avacopan-for-anca-associated-vasculitis-in-a-large-integrated-healthcare-system/