Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Patients receiving B cell depleting therapies (BCDT) for immune mediated inflammatory diseases (IMIDs) have high risk of poor COVID-19 outcomes and strategies for COVID-19 prevention and treatment of this vulnerable group are needed. Pre-exposure prophylaxis with tixagevimab/cilgavimab (Evusheld©) has been available under FDA-Emergency Use Authorization in the U.S. since December 2021. As of January 18, 2022 the Cleveland Clinic has made tixagevimab/cilgavimab available to patients receiving BCTD and other select high risk patients. Unknown at present is how effective this preventative strategy will be in the real world. Here were report our experience with COVID-19 breakthrough despite tixagevimab/cilgavimab.
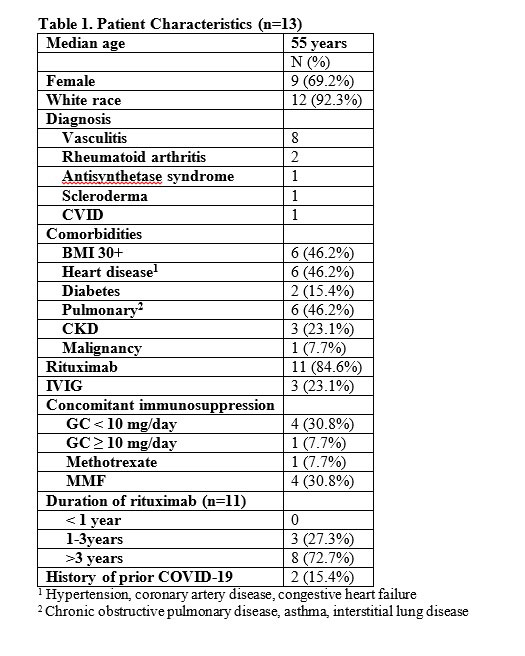
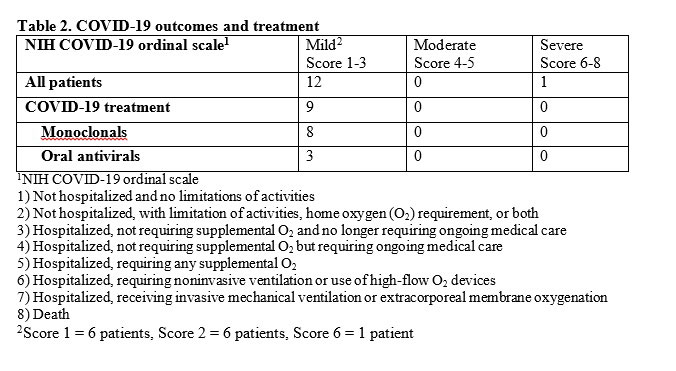
Methods: All pharmacy records from within a large health care system were electronically searched for patients who met criteria to receive tixagevimab/cilgavimab as defined by the Cleveland Clinic COVID-19 Pharmacy & Therapeutics sub-committee, and then subsequently diagnosed with COVID-19. From the curated list of breakthrough patients of interest, electronic records were manually reviewed to extract data on COVID-19 infection, vaccination and outcomes as assessed by an 8 point NIH ordinal scale, as defined in Table 2.
Results: A total of 417 patients with IMIDs received tixagevimab/cilgavimab across the rheumatology (n=261), allergy/immunology (n=78) and neurology (n=78) departments between January18. 2022 and May 28, 2022. From this cohort, 13 patients (3%) experienced a breakthrough COVID-19 infection after receiving at least one dose of tixagevimab/cilgavimab (Table 1). All patients had been vaccinated against COVID-19. 6/13 patients developed infection a median of 19 days (13-84) after receiving 150 mg/150 mg of tixagevimab/cilgavimab. 7/10 patients developed infection a median of 34 days (19-72) after either single dose of 300 mg/300 mg or after their second dose of 150/150 mg. Overall 12 patients had a mild course and recovered at home (Table 2). 1 patient was hospitalized and required high flow oxygen and there were no deaths.
Conclusion: This early experience suggests that COVID-19 infection after tixagevimab/cilgavimab occurs infrequently and is mild in severity, but further larger prospective studies are needed.
To cite this abstract in AMA style:
Calabrese C, Kirchner E, villa forte a, Hajj-Ali R, Langford C, Fernandez j, Carlson A, Moss B, Sayles V, Pallotta A, kim A, Calabrese L. Early Experience of Breakthrough COVID-19 Infections in Patients Who Received Pre-exposure Prophylaxis with Tixagevimab/cilgavimab [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/early-experience-of-breakthrough-covid-19-infections-in-patients-who-received-pre-exposure-prophylaxis-with-tixagevimab-cilgavimab/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-experience-of-breakthrough-covid-19-infections-in-patients-who-received-pre-exposure-prophylaxis-with-tixagevimab-cilgavimab/