Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients (pts) with PsA experience lower quality of life than both the general population and pts with psoriasis alone1,2. Recent PsA treatment recommendations highlight the importance of maximizing long‐term (LT) health‐related quality of life (HRQoL) and social participation as primary goals of therapy3. We aimed to determine whether early clinical improvement with guselkumab (GUS) predicts future attainment of enhanced HRQoL.
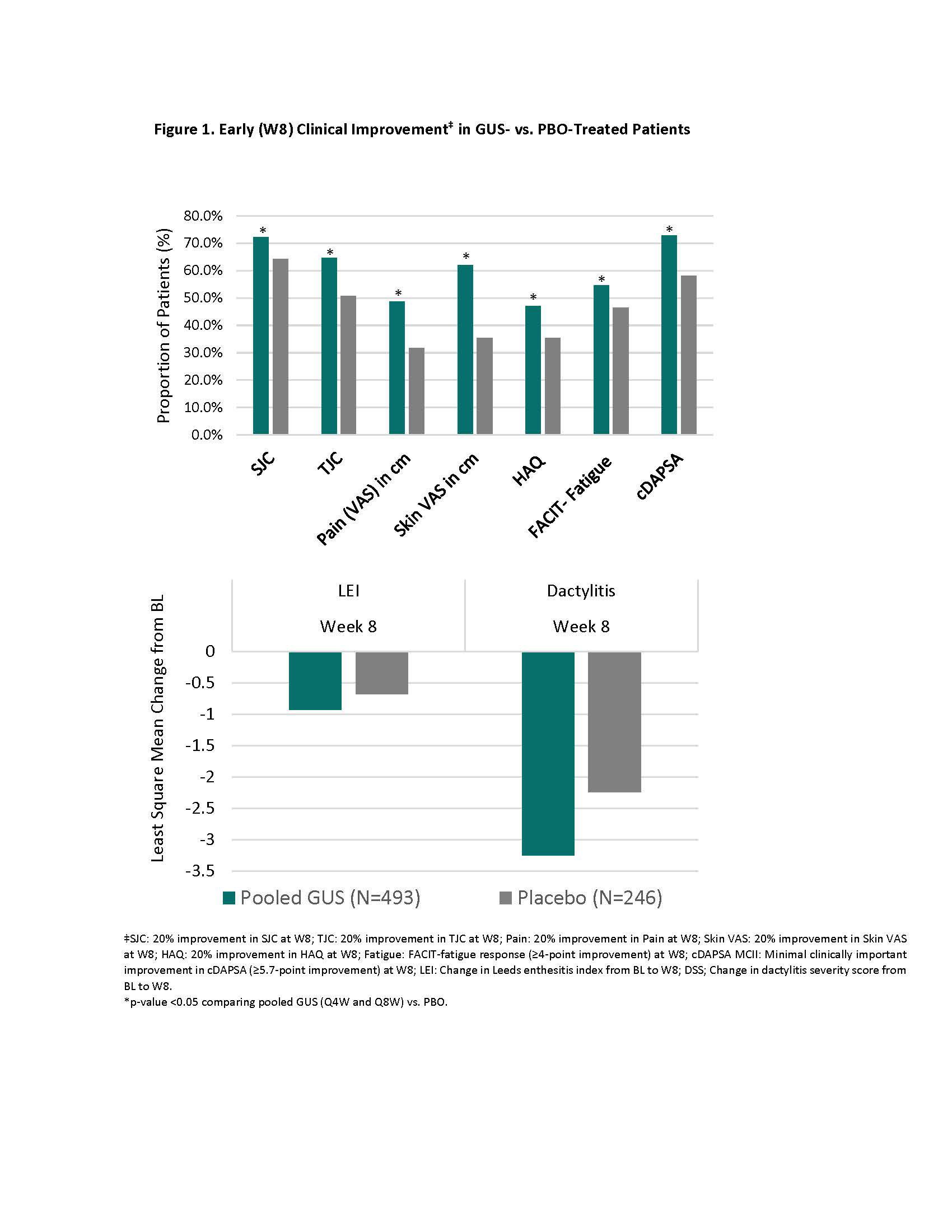
Methods: DISCOVER-2 enrolled adults naïve to biologics/JAK inhibitors who had active PsA defined as swollen joint count (SJC) ≥5, tender joint count (TJC) ≥5, and CRP ≥0.6 mg/dL. 739 pts were randomized (1:1:1) and treated with GUS 100 mg every 4 weeks (Q4W; n=245); GUS 100 mg at W0, W4, then Q8W (n=248); or placebo (PBO) (n=246). In this post hoc analysis, pts treated with GUS Q4W and GUS Q8W were pooled. Early (W8) clinical improvement was defined as any of: (i) ≥20% improvement in SJC, TJC, pt pain, pt skin visual analog scale (VAS), and HAQ-DI; (ii) ≥4-point improvement in the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score; (iii) minimally clinically important improvement (MCII) in clinical disease activity in PsA (cDAPSA; ≥5.7 points); (iv) change in Leeds enthesitis index (LEI) and dactylitis severity score (DSS) at W8 among pts with BL enthesitis and dactylitis, respectively. Time-averaged changes in HRQoL estimates from BL to W52-100 were determined for Dermatology Life Quality Index (DLQI), EQ-5D Index & VAS, and SF-36 mental (MCS) and physical (PCS) component summary scores. The association between early clinical improvement at W8 and LT HRQoL among GUS-treated pts was assessed with mixed models.
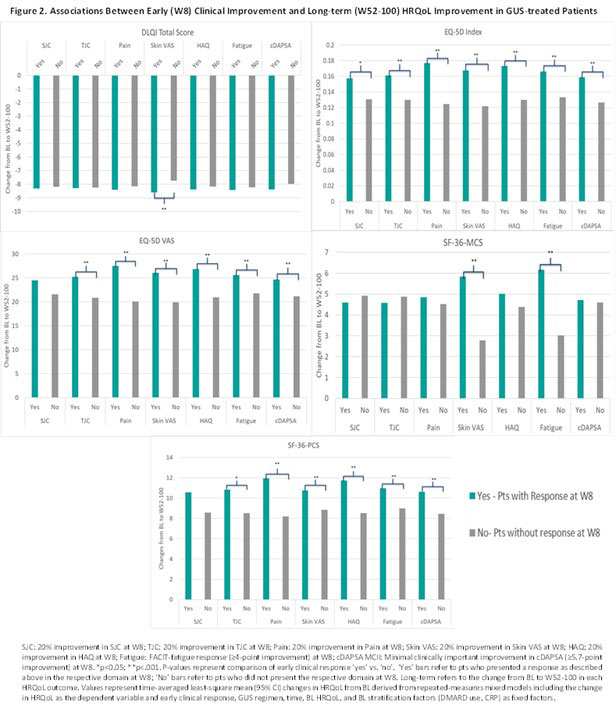
Results: Clinical improvement by W8 was significantly greater among GUS-treated patients compared with PBO (Fig 1). Early clinical improvement with GUS was associated with greater increase in HRQoL (EQ-5D) at W52 through W100, with the exception of SJC on EQ-5D VAS (Fig 2; LEI & DSS results not shown). Similarly, pts achieving early clinical improvement in any domain except DSS and SJC experienced significantly greater benefits in physical function (SF-36 PCS) at W52-W100. Early improvements in skin disease and fatigue were associated with greater improvement in mental health (SF-36 MCS) at W52-W100, while for skin-specific HRQoL (DLQI), early pt skin VAS response was the only predictor of HRQoL at W52-W100. Although significantly lower than in pts with early clinical improvement, benefits in HRQoL were also observed in pts without clinical improvement at W8.
Conclusion: Clinical response at W8 with GUS was associated with significantly greater improvements in HRQoL from W52 through W100. Although pts without early clinical improvement demonstrated benefits in LT HRQoL, early response in distinct PsA domains differentially impacted more specific aspects of HRQoL over 2 years. In contrast, significantly greater improvements in overall and physical HRQoL were observed among responders across several PsA domains.
References:
1. Michelsen B et al. Ann Rheum Dis. 2018 Sep;77(9):1290-4.
2. Rosen CF, et al. Rheumatology (Oxford). 2012 Mar;51(3):571-6.
3. Coates LC et al. J Rheumatol. 2022 Mar;jrheum.211331.
To cite this abstract in AMA style:
McInnes I, Soriano E, Tam L, Shiff N, Shawi M, Rampakakis E, Curtis J. Early Clinical Improvement as Predictor of Long-term Health-Related Quality of Life in Psoriatic Arthritis Patients Treated with Guselkumab: Post Hoc Analysis Through 2 Years of a Phase 3 Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/early-clinical-improvement-as-predictor-of-long-term-health-related-quality-of-life-in-psoriatic-arthritis-patients-treated-with-guselkumab-post-hoc-analysis-through-2-years-of-a-phase-3-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-clinical-improvement-as-predictor-of-long-term-health-related-quality-of-life-in-psoriatic-arthritis-patients-treated-with-guselkumab-post-hoc-analysis-through-2-years-of-a-phase-3-study/