Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment (0985–0989)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Up to 85% of patients with SLE experience skin disease.1 The Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI) is a validated index to measure skin disease severity; activity scores (CLASI-A) range from 0 (mild) to 70 (severe) and include measures for erythema, scale/hypertrophy, mucous membrane lesions, recent hair loss, and nonscarring alopecia. In the phase 3 TULIP-1 and -2 trials of patients with SLE, a greater proportion of patients with CLASI-A ≥10 at baseline achieved ≥50% CLASI-A reduction at Week 12 with anifrolumab compared with placebo.2,3 We further evaluated the effect of anifrolumab on skin-specific SLE disease activity using data pooled from TULIP-1 and -2.
Methods: TULIP-1 and -2 were 52-week, randomized, double-blind, placebo-controlled trials that evaluated the efficacy and safety of anifrolumab (300 mg IV every 4 weeks for 48 weeks) in patients with moderately to severely active SLE despite standard-of-care treatment. TULIP-1 and -2 were analyzed separately using restricted medication rules per the TULIP-2 protocol, and data from both trials were pooled. We compared skin responses over time in patients receiving anifrolumab vs placebo. A CLASI-A response was defined as ≥50% reduction of CLASI-A from baseline for patients with CLASI-A ≥10. Baseline CLASI-A >0 as well as ≥75% reduction were also evaluated. Time to CLASI-A response sustained to Week 52 was evaluated using a Cox proportional hazards model.
Results: In total, 360 patients received anifrolumab and 366 received placebo. At baseline, 95.9% (696/726) of patients had CLASI-A >0, and 27.7% (201/726) had CLASI-A ≥10 (balanced between groups). In the subgroup of patients with baseline CLASI-A ≥10, CLASI-A response (≥50% reduction) was achieved by Week 12 in 46.0% (49/107) of patients receiving anifrolumab vs 24.9% (24/94) receiving placebo (difference 21.0; 95% CI 8.1%, 34.0%; nominal P< 0.001) (Figure 1). Separation between treatment groups was observed as early as Week 8 (difference 14.3; 95% CI 1.8%, 26.9%; nominal P< 0.02) (Figure 1). Time to CLASI-A response sustained to Week 52 favored anifrolumab in TULIP-1 (hazard ratio [HR] 1.91; 95% CI 1.14, 3.27) and TULIP-2 (HR 1.55; 95% CI 0.87, 2.85) (Figure 2). Of the subgroup of patients with baseline CLASI-A >0, a greater number of patients achieved a CLASI-A response (≥50% reduction) by Week 12 in the anifrolumab vs placebo groups in both TULIP-1 and -2 (nominal P< 0.05) (Figure 3); similar effects were observed in the subgroup of patients with baseline CLASI-A ≥10 in both TULIP-1 and -2 (nominal P< 0.05) (Figure 3).
Conclusion: Anifrolumab treatment was associated with rapid and durable improvements in skin-specific SLE disease activity, as assessed by CLASI-A, in subgroups of patients with mild to severe baseline cutaneous disease activity. These findings support the potential of anifrolumab to reduce skin disease activity in patients with moderately to severely active SLE.
References
- Rothfield N. Clin Dermatol. 2006;24:348–62.
- Furie RA. Lancet Rheumatol. 2019;1:e208–19.
- Morand EF. N Engl J Med. 2020;382:211–21.
Writing assistance by Rebecca Jones, PhD (JK Associates Inc., a Fishawack Health Company).
This study was sponsored by AstraZeneca.
 Figure 1. Percentage of Patients With CLASI-A ≥10 at Baseline Achieving ≥50% Reduction in CLASI-A From Baseline Over Time in Pooled Data From the TULIP-1 and TULIP-2 Trials
Figure 1. Percentage of Patients With CLASI-A ≥10 at Baseline Achieving ≥50% Reduction in CLASI-A From Baseline Over Time in Pooled Data From the TULIP-1 and TULIP-2 Trials
 Figure 2. Time to CLASI-A Response (≥50% Reduction From Baseline) Sustained to Week 52 in Patients With CLASI-A ≥10 at Baseline in Data From the TULIP-1 and TULIP-2 Trials
Figure 2. Time to CLASI-A Response (≥50% Reduction From Baseline) Sustained to Week 52 in Patients With CLASI-A ≥10 at Baseline in Data From the TULIP-1 and TULIP-2 Trials
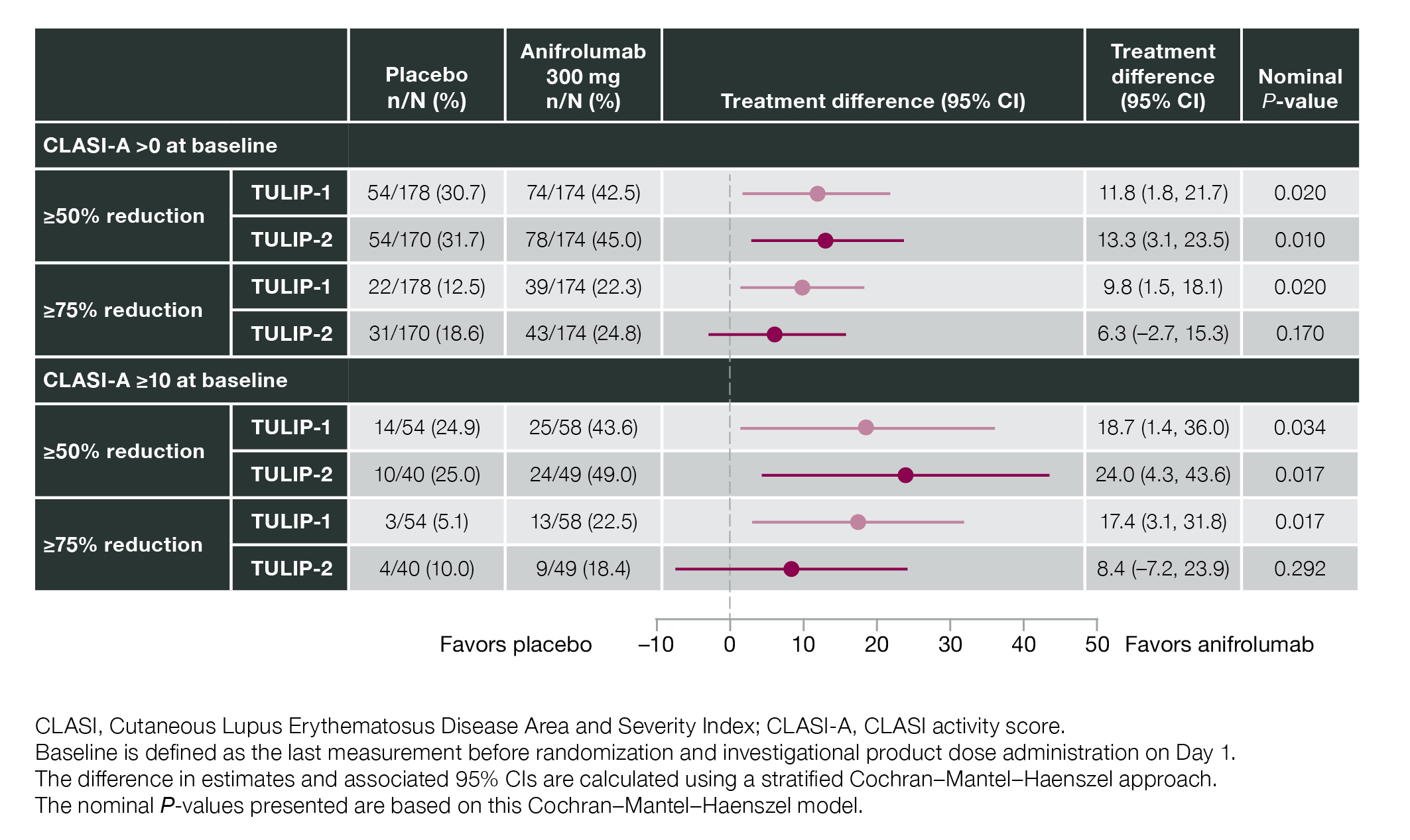 Figure 3. CLASI-A Response at Week 12 by Baseline CLASI-A at 50% and 75% Response Thresholds in Data From the TULIP-1 and TULIP-2 Trials
Figure 3. CLASI-A Response at Week 12 by Baseline CLASI-A at 50% and 75% Response Thresholds in Data From the TULIP-1 and TULIP-2 Trials
To cite this abstract in AMA style:
Werth V, Furie R, Morand E, Kahlenberg J, Kalyani R, Abreu G, Pineda L, Tummala R. Early and Sustained Reduction in Severity of Skin Disease with Anifrolumab Treatment in Patients with Active SLE Measured by the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI): Pooled Data from 2 Phase 3 Studies [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/early-and-sustained-reduction-in-severity-of-skin-disease-with-anifrolumab-treatment-in-patients-with-active-sle-measured-by-the-cutaneous-lupus-erythematosus-disease-area-and-severity-index-clasi/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/early-and-sustained-reduction-in-severity-of-skin-disease-with-anifrolumab-treatment-in-patients-with-active-sle-measured-by-the-cutaneous-lupus-erythematosus-disease-area-and-severity-index-clasi/
