Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Remission is increasingly becoming a treatment goal in rheumatoid arthritis (RA) patients and DAS28 remission criteria are widely used, despite their limitations.
The purpose of this study was to study frequency, duration, and timing of sustained remission (SR) defined as DAS<2.6 for at least 6 months, and to compare efficacy of different therapies, in patients with established RA treated with Tumor Necrosis Factor inhibitors (aTNF) in the observational setting of Southern Sweden.
Methods:
aTNF treatments in RA patients registered in the SSATG register were eligible for this study. Remission time was defined as time between first visit after treatment initiation with DAS28<2.6 and subsequent visit with DAS28>2.6.
The comparison of the drugs was done using logistic regression analysis, adjusting for baseline variables, and the ORs for achieving SR 12 and 24 months were adjusted for baseline age, disease duration, DAS28, HAQ, concomitant MTX and prednisolone. Life-table techniques were used to estimate SR time, looking only on first biologic treatments.
Results:
Of 3446 initiated treatments, 481 (14 %) fulfilled the criteria. Of those who reached SR 63.2% did so within the first year.
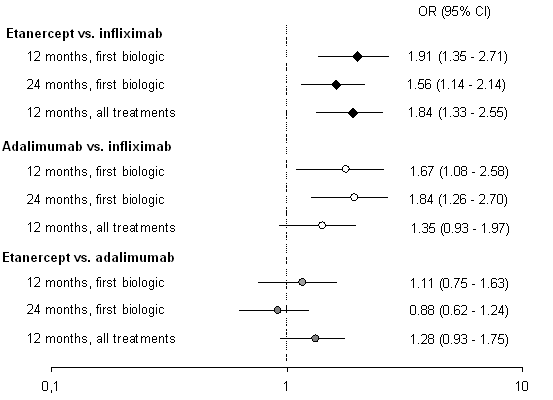
Among patients naïve to biologic treatments and after correction for baseline variables, the ORs for achieving SR within the first 12 months of treatment were 1.9 for etanercept and 1.7 for adalimumab, with infliximab as the reference drug, while etanercept and adalimumab were not significantly different, see figure 1.
Median estimated SR survival time was 3.9 years (etanercept 4.4y, adalimumab 4.8y and infliximab 3.0y). Estimated SR survival was significantly longer for etanercept and adalimumab compared to infliximab (p < 0.05), with no significant difference between etanercept and adalimumab. The estimated SR survival, at 12, 24, and 48 months was 94%, 68% and 48% respectively. After 120 months 11% remained in SR.
Conclusion:
Differences were seen in remission rates and duration between different medications. Within 12 months etanercept and adalimumab had significantly higher likelihood of reaching remission than infliximab, and the estimated remission survival was significantly longer for etanercept and adalimumab compared to infliximab.
Figure 1. Odds of having achieving sustained remission on first biologic after 12 and 24 months, and for all treatments after 24 months. Drug on right the reference drug.
Adjusted for age, sex, disease duration, concomitant methotrexate and prednisolone treatment, CRP, DAS28 and HAQ score at baseline.
Disclosure:
J. T. Einarsson,
None;
P. Geborek,
None;
T. Saxne,
None;
M. C. Kapetanovic,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/duration-of-sustained-remission-and-differences-in-responce-between-medications-in-tumor-necrosis-factor-inhibitor-treated-rheumatoid-arthritis-patients/