Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Synthetic glucocorticoids (steroids) are recommended for and commonly used in rheumatoid arthritis (RA) as initial “bridge therapy”, acting to rapidly reduce the inflammatory response and associated symptoms. Current treatment guidelines in several countries recommend short-term use at a low dose, and tapered quickly due to side effect profile. Steroids are given as oral alone and often combined with parenteral (intramuscular and intra-articular) formulations; patients may receive both to control RA inflammation, perhaps with the intent of being able to shorten the duration of exposure to steroids. The objective of this study was to compare duration of oral steroid exposure among those who receive oral monotherapy to those who receive both oral and parenteral formulations.
Methods: Data were from patients with classifiable early RA enrolled in a nationwide real-world early rheumatoid arthritis cohort, with at least 24 months of follow-up. Patients were stratified based on steroid use (oral only vs combination parenteral and oral, regardless of DMARD exposure) within the first 3 months, to account for a potential lag time for the treating rheumatologist to decide steroid initiation was necessary. Persons who already received steroids before study entry were excluded, to avoid prevalent user bias. Parenteral use without an oral steroid was not considered in this analysis, due to an inability to reliably ascertain duration of exposure from an injection. Kaplan-Meier survival curves were constructed to compare steroid persistence time between oral and combination routes of administration, and differences between curves were tested using the log-rank test.
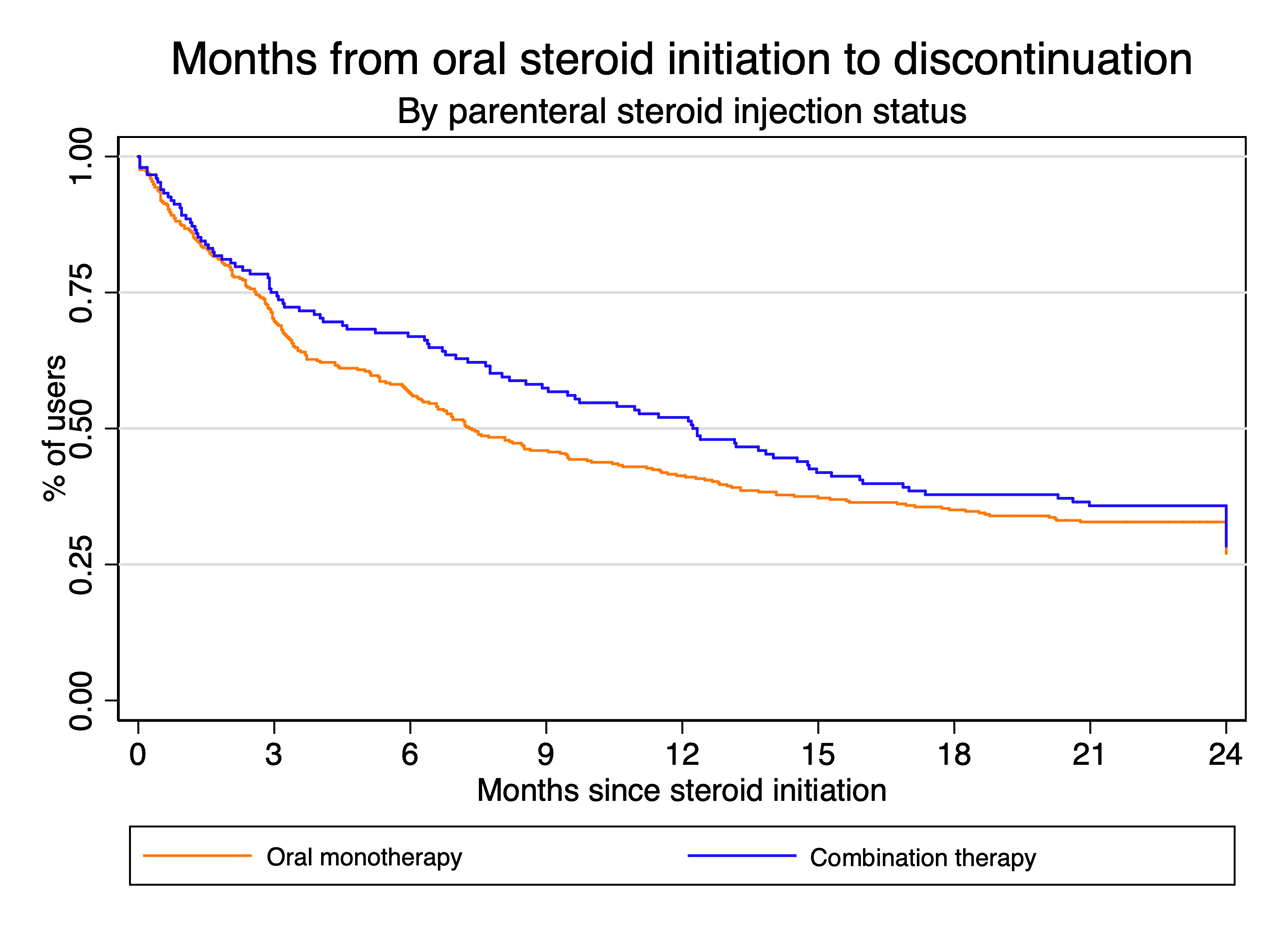
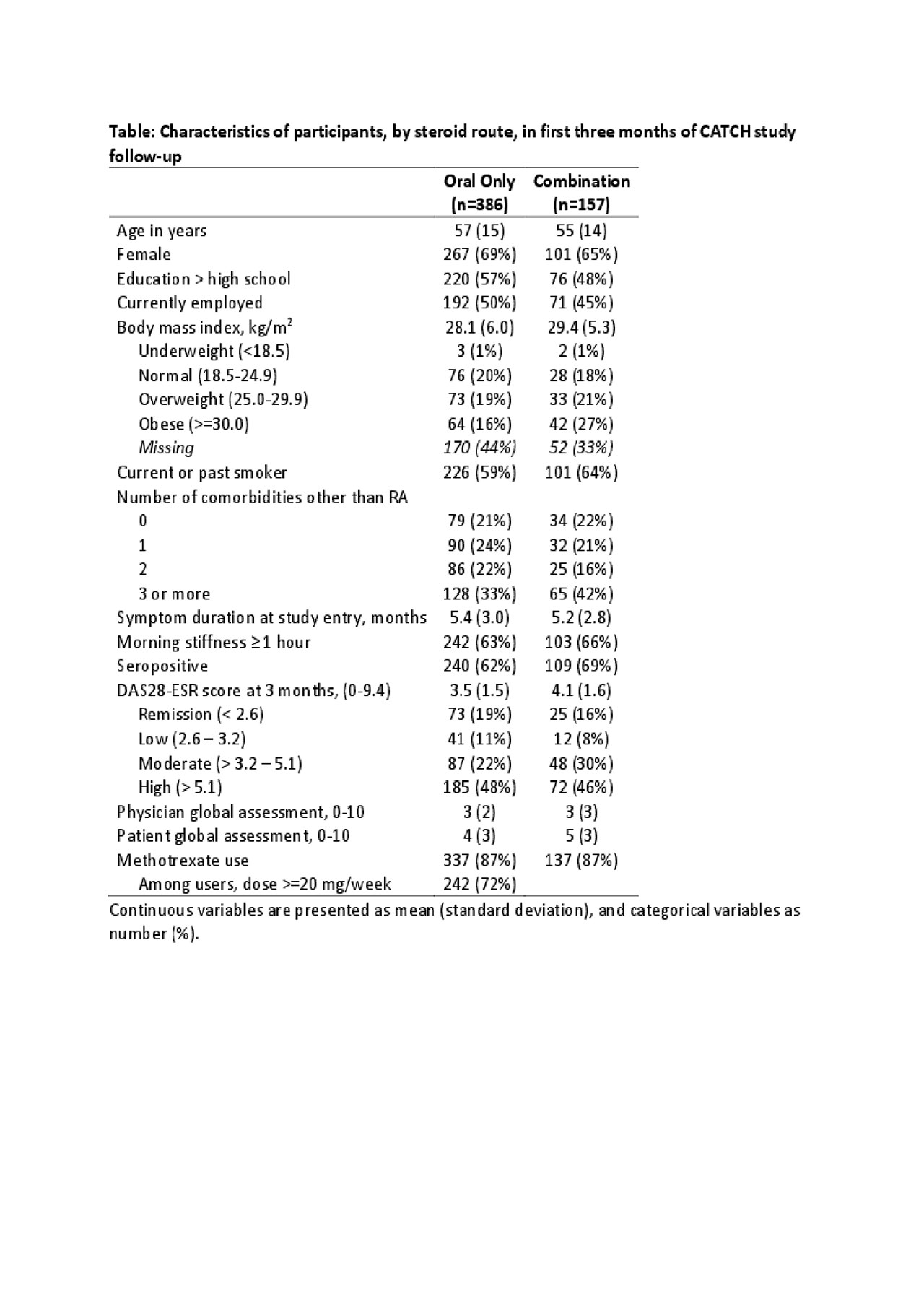
Results: Of 1,573 participants recruited to the overall study between 2007-2017 with at least 24 months of follow-up, 533 (35%) received either oral or combination steroids. Combination steroid users were less likely to be female, to have a higher education and to be currently employed (Table). Disease activity at 3 months was highest in the combination steroid groups, as were the physician and patient global assessments of disease. The median duration of oral steroid use did not differ significantly between those receiving oral or combination steroids when parenteral steroid injections were given within the first 3 months of follow-up (7 months oral monotherapy versus 11 months combination, Mann-Whitney U test p-value = 0.20). Throughout follow-up, the discontinuation patterns were similar (Figure), and there was no difference in Kaplan-Meier survival curves (p-value = 0.29).
Conclusion: Combination steroids were used in people with more active disease, but combining oral and parenteral steroids did not reduce the duration of oral steroid treatment in this prospective observational cohort compared with those receiving oral steroids alone nor did they reduce disease activity more so than oral steroids. The subset of patients receiving combination steroids may have more persistent disease, and may benefit from closer monitoring and use of more intensive DMARD or other advanced therapeutic strategies.
To cite this abstract in AMA style:
Andersen K, Schieir O, Valois M, Bartlett S, Bessette L, Boire G, Hazlewood G, Hitchon C, Keystone E, Pope J, Tin D, Thorne C, Bykerk V, (CATCH) Investigators C. Duration of Oral Corticosteroid Therapy Does Not Change with the Addition of a Parenteral Injection: Results from a Real-World Canadian Early RA Cohort [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/duration-of-oral-corticosteroid-therapy-does-not-change-with-the-addition-of-a-parenteral-injection-results-from-a-real-world-canadian-early-ra-cohort/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/duration-of-oral-corticosteroid-therapy-does-not-change-with-the-addition-of-a-parenteral-injection-results-from-a-real-world-canadian-early-ra-cohort/