Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: APS ACTION is a multidisciplinary, international research network focused on developing collaborative studies to improve antiphospholipid syndrome (APS) diagnosis and management. There is limited prospective evidence guiding the optimal duration of anticoagulation in thrombotic APS, particularly across varied antibody profiles, levels, and thrombosis risk factors. The objective of this survey was to evaluate clinical decision-making practices among APS ACTION members regarding the duration of anticoagulation for secondary thrombosis prevention in APS, using standardized clinical scenarios.
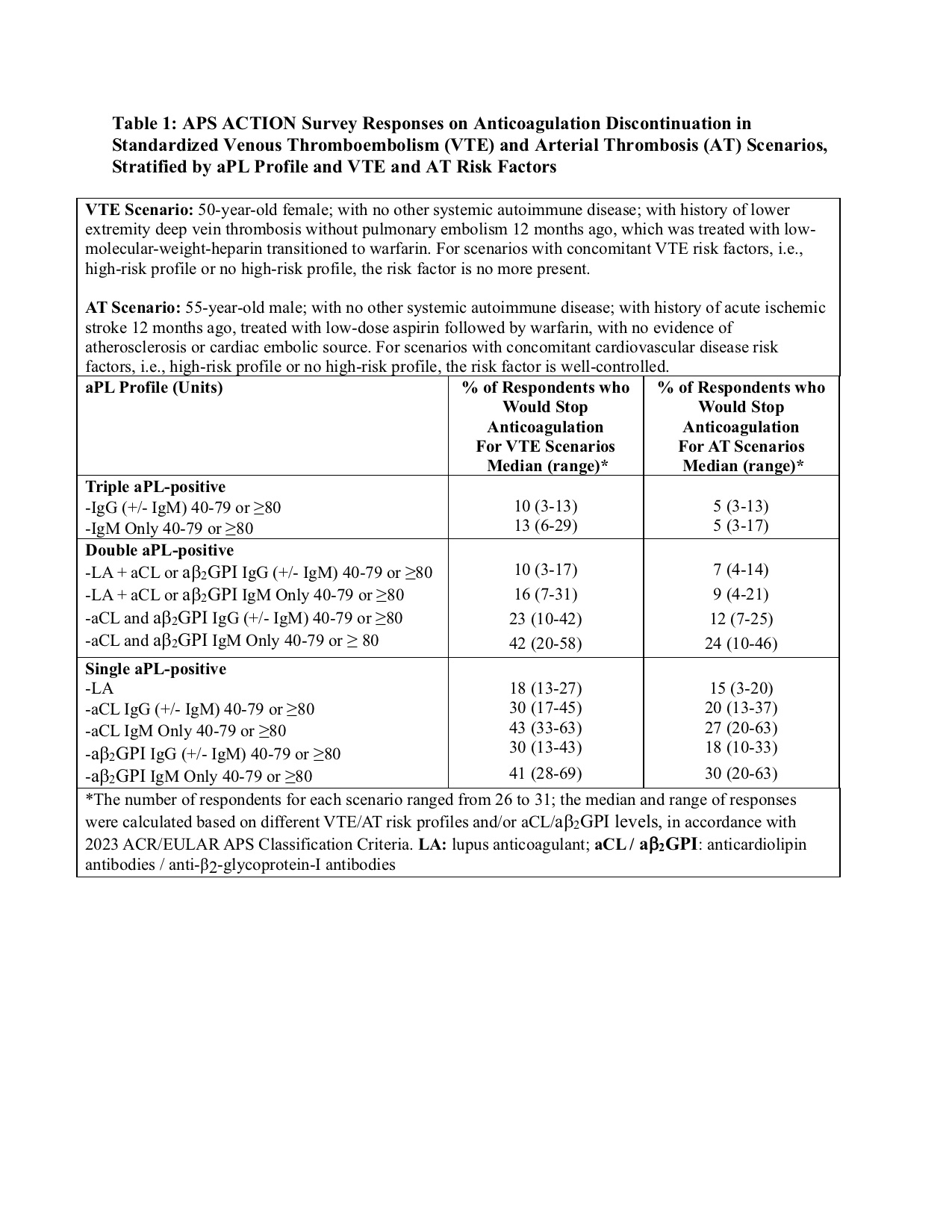
Methods: A cross-sectional electronic survey was distributed to APS ACTION members in April 2025. Participants were asked to assess whether they would discontinue long-term anticoagulation in patients with different scenarios designed, based on the clinical and laboratory domains of the 2023 ACR/EULAR APS Classification Criteria. Each scenario was stratified by aPL profile (triple, double, or single-positive), aCL/aβ2GPI isotype (IgG +/-IgM, or IgM only), and aCL/aβ2GPI level (40–79U or ≥80U), as well as by venous thromboembolism (VTE) and arterial thrombosis (AT) risk profiles (Table 1). Descriptive statistics summarize the proportion of clinicians favoring discontinuation across scenarios.
Results: Fifty-nine APS ACTION members involved in patient care received the survey, and 31 (53%) (mean years in practice 25 ± 12, rheumatologists: 21 [68%], hematologists: 3 [10%],and other: 7 [22%]) responded. When stratified by aPL profile, anticoagulation discontinuation rates were the lowest in triple aPL-positive patients, with only 10–16% willing to stop in different VTE and 7% in AT scenarios. In double aPL-positive patients, discontinuation rates were lower for lupus anticoagulant (LA)-positivity, and higher when LA was negative, particularly for IgM-only profiles (10-42% would stop anticoagulation for different VTE and 7-28% for AT profiles). Single aPL-positive patients demonstrated the highest likelihood of discontinuation (lowest for LA-positive patients); depending on the aPL and thrombosis risk profiles, rates of discontinuation of anticoagulation were 13-69% for VTE and 3-63% for AT (Table 1).
Conclusion: Among APS ACTION members, anticoagulation discontinuation decisions in thrombotic APS vary significantly depending on the aPL profile, thrombosis type, and concomitant thrombosis risk factors at the time of the event. Clinicians were more likely to stop anticoagulation in VTE than AT scenarios, in patients with IgM-only profiles, and in the absence of a positive LA test. These findings highlight areas of variability in clinical practice and underscore the need for risk-stratified controlled studies to inform anticoagulation duration in diverse APS phenotypes.
To cite this abstract in AMA style:
Erton Z, Vega J, Cohen H, Erkan D. Duration of Anticoagulation in Antiphospholipid Antibody-positive Patients: Results from an AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Survey [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/duration-of-anticoagulation-in-antiphospholipid-antibody-positive-patients-results-from-an-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-survey/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/duration-of-anticoagulation-in-antiphospholipid-antibody-positive-patients-results-from-an-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-survey/