Session Information
Date: Monday, November 9, 2015
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
There is limited information on drug
survival (i.e., continuation versus discontinuation of drug treatment) of RA patients
who received a 2nd bDMARD therapy after 1st anti-TNF
therapy. The objective of this study was to compare continuation, discontinuation,
re-start and switch rates of RA patients who received an anti-TNF versus a
non-anti-TNF as 2nd bDMARD.
(AOK PLUS) that included all insured RA patients (at least one RA diagnosis:
ICD-10 M05 or M06; aged >18 years). RA patients were included if they
received at least one anti-TNF and, additionally, one 2nd bDMARD
(anti-TNF or non-anti-TNF) during 01/01/2010–31/12/2012 with a requested
follow-up of at least 12 months.
patients who discontinued (treatment gap >90 days), switched to a 3rd
bDMARD, re-started (at least one prescription of the 2nd bDMARD
after discontinuation) or continued therapy during a 12-month follow-up were
analyzed.
regression model, adjusting for baseline confounding variables (age, sex, Charlson
Comorbidity Index [CCI], prior and concomitant medications, anti-TNF/non-anti-TNF
as 2nd biologic treatment), was used to assess factors associated
with discontinuation or switch (combined outcome, irrespective of a later
re-start) of 2nd bDMARD. In all analyses, patients who had received rituximab
(RTX) as a 2nd bDMARD were excluded because this agent is not given
on a continuous basis after the initial two doses.
RA patients received at least one prescription of an anti-TNF. Of these, 451
patients received at least one prescription of a 2nd non-RTX bDMARD
(340 anti-TNF: 116 adalimumab, 42 certolizumab, 120 etanercept, 46 golimumab,
16 infliximab; 111 non-anti-TNF: 40 abatacept, 3 anakinra, 68 tocilizumab).
Mean age of the anti-/non-anti-TNF groups was 52.6/55.9 years (p=0.053) and
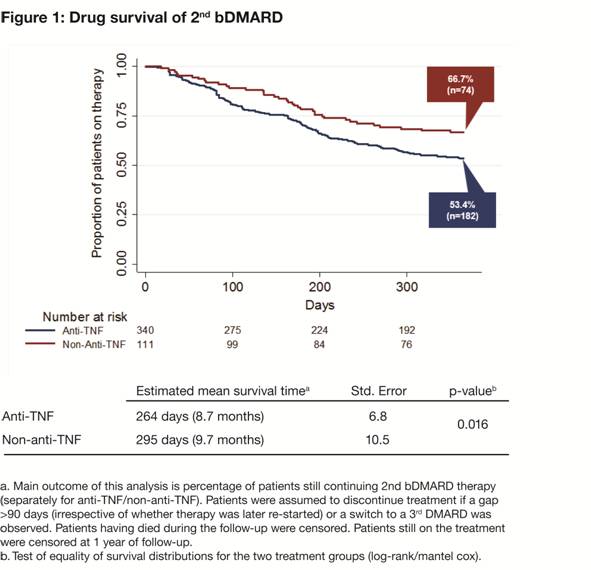
77.4/79.3% were female (p=0.792), respectively. After 12 months, 53.4% of patients
receiving a 2nd anti-TNF vs 66.7% (p=0.016) receiving a 2nd
non-anti-TNF continued their therapy, 3.8 vs 1.8% (p=0.387) re-started their
therapy after discontinuation, 14.1 vs 19.8% (p=0.179) discontinued the therapy
without re-start, and 28.7 vs 11.7% (p<0.001) had switched to a 3rd
bDMARD. Figure 1 presents the Kaplan–Meier curves for the two patient groups
showing time to switch or discontinuation.
independent variables significantly associated with earlier therapy
discontinuation or switch were higher CCI (hazard ratio [HR]=1.127 per CCI
score point; 95% CI: 1.036, 1.226), concomitant gout medication (HR=1.444; 95%
CI: 1.046, 1.993) and prescription of an anti-TNF as 2nd bDMARD
(HR=1.513; 95% CI: 1.052, 2.175).
Conclusion: Our results suggest that patients are at higher risk
of treatment discontinuation or switch to a 3rd bDMARD after 12
months if they have received an anti-TNF versus a non-anti-TNF as 2nd
bDMARD therapy.
Disclosure: T. Wilke, LEO Pharma, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Boehringer Ingelheim, Merck, Abbvie, Pharmerit, 9; S. Mueller, Bristol-Myers Squibb, 5; I. Majer, Bristol-Myers Squibb, 5; M. Heisen, Bristol-Myers Squibb, 5; A. Fuchs, None; U. Maywald, None.
To cite this abstract in AMA style:
Wilke T, Mueller S, Majer I, Heisen M, Fuchs A, Maywald U. Drug Survival of Second Biologic DMARD Therapy in Patients with Rheumatoid Arthritis: Comparison of a Second Anti-TNF with a Second Non-Anti-TNF after Discontinuation of a First Anti-TNF [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/drug-survival-of-second-biologic-dmard-therapy-in-patients-with-rheumatoid-arthritis-comparison-of-a-second-anti-tnf-with-a-second-non-anti-tnf-after-discontinuation-of-a-first-anti-tnf/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-survival-of-second-biologic-dmard-therapy-in-patients-with-rheumatoid-arthritis-comparison-of-a-second-anti-tnf-with-a-second-non-anti-tnf-after-discontinuation-of-a-first-anti-tnf/
