Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Ustekinumab and secukinumab are two new Biologic Disease-modifying Antirheumatic Drugs (bDMARDs) in severe psoriatic arthritis (PsA), targeting respectively IL12-23 and IL 17. Data in real-world are missing for these treatments. For ustekinumab, there is only one study with a large number of patients (160). For secukinumab, there are only the data from clinical trials. The objective was to assess drug survival, efficacy and remission of ustekinumab (UST) and secukinumab (SEK) in a retrospective multicentric cohort of 161PsA.
Methods: This is a multicentric retrospective study of patients suffering from PsA (CASPAR criteria) from July 2011 to April 2018. Drug survival is defined as the time from initiation to discontinuation (stop/switch) of biologic therapy on the registry. Using Kaplan-Meier survival curves and Cox-regression analyses [hazard ratios (HR) and 95% confidence intervals (CIs)], time to discontinuation was compared across the cohort. For peripheral forms, treatment was considered to be effective for patients with a favourable expert opinion or > 30% clinical improvement of swollen and tender joint counts (SJC and TJC). For axial forms, efficacy criteria were: improvement of BASDAI by at least 2 points on a scale from 0 to 10 or 50% improvement (BASDAI 50) or expert opinion. Remission was considered if TJC ≤1, SJC ≤ 1, PASI≤ 1, patient Visual Analogue Scale (VAS) ≤15 , Patient global activity VAS ≤20 and Tender entheseal points≤1 (Very Low Disease Activity (VLDA) criteria except HAQ).
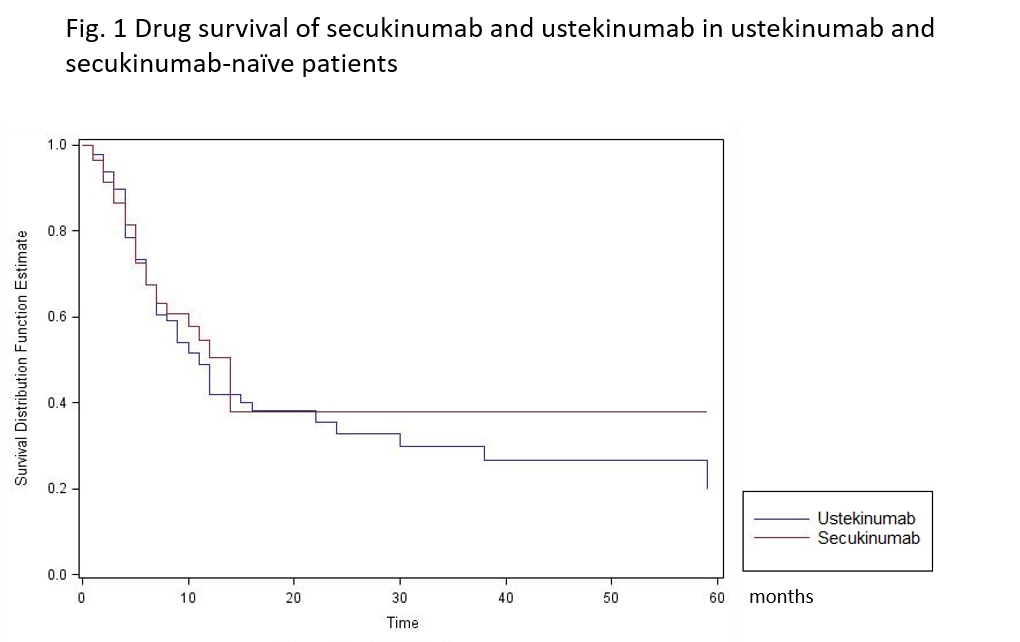
Results: 161 were included with a mean follow up greater than or equal to 6 months. The sex ratio was balanced with 54.7% of women. The mean age was 50.2 years old and the body mass index (BMI) was 27.6 kg/m². The disease duration was 9.6 years. 47.7% of patients did not smoke. The patients presented axial PsA in 59.0%, peripheral PsA in 94.9% and enthesitis in 31.4%. Patients were bDMARD-naïve in 13.0%. The median drug survivals for UST AND SEK were respectively 11 and 12 months. There was no impact of the age, the sex, the disease duration, smoking status or the BMI on the drug survival. The drug survival was similar in UST and SEK-naïve patients (HR, 1.07 (0.67 to 1.70), p=0.77) (Fig 1) as efficacy for both treatments (p=0.15) whereas remission was higher in SEK group (36.2% vs 18.2%, p=0.012).
Conclusion: This is the first real-world study which compares these two new treatments in psoriatic arthritis. Ustekinumab and secukinumab in psoriatic arthritis have similar drug survival and efficacy in our study. However, remission based on VLDA criteria was achieved more often with secukinumab.
To cite this abstract in AMA style:
Letarouilly JG, Sellam J, Richette P, Dieude P, Claudepierre P, Pascart T, Houvenagel E, Guyot MH, Segaud N, Coquerelle P, Maury F, Marguerie L, Deprez X, Salmon JH, Baudens G, Gervais E, Kyheng M, Paccou J, Flipo RM. Drug Survival of Non TNF Inhibitors Bdmards in Psoriatic Arthritis (Ustekinumab/Secukinumab) : A Real-Word Multicentric Cohort of 161 Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/drug-survival-of-non-tnf-inhibitors-bdmards-in-psoriatic-arthritis-ustekinumab-secukinumab-a-real-word-multicentric-cohort-of-161-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-survival-of-non-tnf-inhibitors-bdmards-in-psoriatic-arthritis-ustekinumab-secukinumab-a-real-word-multicentric-cohort-of-161-patients/