Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: CT-P13 is the first biosimilar of the reference infliximab (IFX) prescribed for rheumatoid arthritis, ankylosing spondylitis (AS), Crohn’s disease, ulcerative colitis, psoriasis, and psoriatic arthritis. There are few studies showing long-term, real-world data of its drug survival or safety.
To evaluate drug retention, efficacy and safety of CT-P13 versus IFX in patients with AS enrolled in the Korean College of Rheumatology Biologics (KOBIO) registry.
Methods: Subjects were registered patients with AS who initiated CT-P13 or IFX between Dec 2012 and Dec 2017. Patients in the two groups were matched via propensity score based on age, gender, and baseline Bath Ankylosing Spondylitis Disease Activity Index (BASDAI). In result, 124 patients were selected in each group. Drug retention, efficacy, and adverse events (AEs) in both groups were assessed over the 4-year follow-up period.
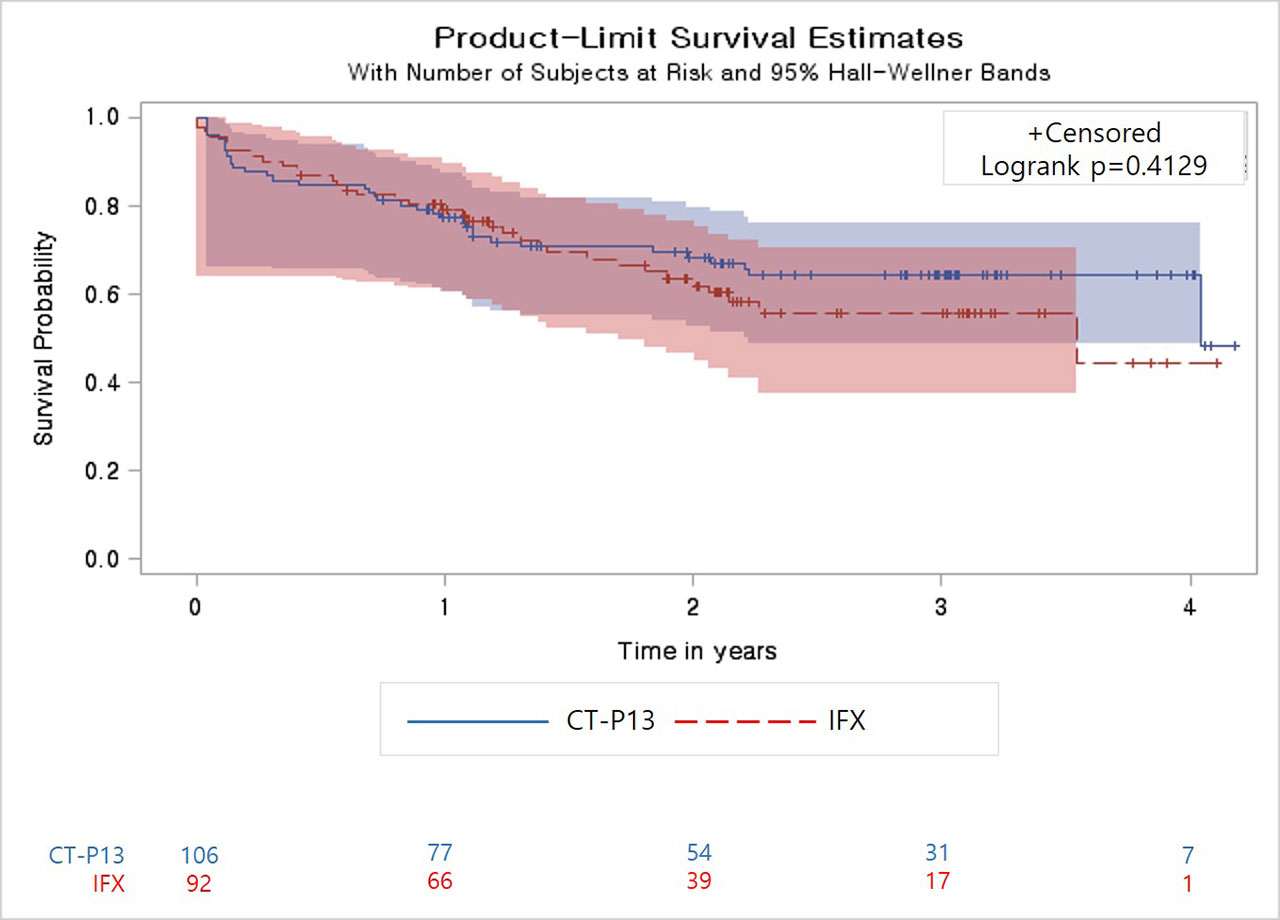
Results: The median treatment duration was 2.1 years in CT-P13, and 1.8 years in IFX treated patients (p = 0.1148). Overall, 79% of patients received each agent as first-line therapy. Drug retention of CT-P13 versus IFX was comparable in the total patient population (p = 0.4129, Figure), for first-line users (p = 0.1532) and second or more lines of users (p = 0.4452). Changing or discontinuing the agent occurred in 31.5% of patients in the CT-P13 group, and 33.1% in the IFX group. The most common reason for change or discontinuation was lack of efficacy (CT-P13 group: 38.5%; IFX group: 24.4%). CT-P13 and IFX users demonstrated comparable improvements in BASDAI, ASDAS-ESR, ASDAS-CRP and the ASDAS improvement criteria. In total, 13 AEs were reported in the CT-P13 group and 17 in the IFX group that led to treatment change or discontinuation.
Conclusion: In this real-world study, long-term data from Korean patients with AS show that CT-P13 has a drug retention rate comparable to IFX, and also similar efficacy along with an acceptable safety profile.
To cite this abstract in AMA style:
Kim H, Lee E, Lee S, Park Y, Shin K. Drug Survival and Safety of Biosimilar CT-P13 versus Reference Infliximab in Patients with Ankylosing Spondylitis: Data from the Korean College of Rheumatology Biologics Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/drug-survival-and-safety-of-biosimilar-ct-p13-versus-reference-infliximab-in-patients-with-ankylosing-spondylitis-data-from-the-korean-college-of-rheumatology-biologics-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-survival-and-safety-of-biosimilar-ct-p13-versus-reference-infliximab-in-patients-with-ankylosing-spondylitis-data-from-the-korean-college-of-rheumatology-biologics-registry/