Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
To evaluate long-term efficacy and
safety of biological disease modifying antirheumatic drug (bDMARD) in
real-life practice and identify risk factors related to remission and drug discontinuation
in rheumatoid arthritis (RA) patients.
Methods:
Two hundred and fifty-six patients
fulfilling 1987 ACR or 2010 ACR/EULAR classification criteria for RA and starting
bDMARD between December 2009 and October 2014 were selected from the RDPA
register. Baseline demographic and clinical data were retrieved. The cumulative
probability of bDMARDs discontinuation over 5 years of follow-up and factors
associated with remission and bDMARDs withdrawals was analyzed.
Results:
Almost half (46%) of patients were
initially treated with rituximab (RTX), followed by etanercept (ETN) 33% and
infliximab (IFX) 21%. Less than 10% of patients were subsequently switched to a
second bDMARD (ETN to RTX 62.5%, IFX to RTX 33.3% and RTX to IFX 4.2%). In
patients who continued using the first bDMARDs, remission had been achieved in
7.2% and 21.5% at 1 year and 5 years, respectively. In multivariate
analysis, the factor predicting remission from first bDMARD was male gender with
hazard ratio (HR) 1.9 (95%CI 1.05-3.45). At 3 years
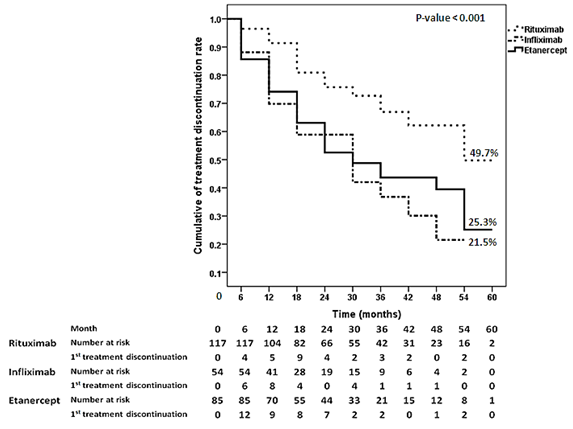
follow-up, the drug survival rates were 67%, 44%, and 37% for RTX, ETN, and
IFX, respectively. The probability of bDMARD continuation during 5 year of
follow-up is shown in figure. In multivariate analysis, RTX was significantly
associated with highest drug survival. Hazard ratio for drug discontinuation were
2.60 (95%CI 1.53-4.42) for IFX and 2.15 (95%CI 1.36-3.42) for ETN compared to RTX.
Thirty-nine percent of patients
stopped treatments, due to inadequate response (42%), serious adverse event
(SAE) (22%), non-adherence (14%), and remission/low disease activity
(13%). SAE comprised of non-mycobacterium infection (32%), mycobacterium
infection (24%) and malignancy (12%). There were 5 deaths (4/5 in RTX
group) because of lung cancer, community-acquired pneumonia, aspiration
pneumonia, hepatitis B virus infection, and sudden cardiac arrest.
Conclusion:
During 5 years of follow-up, 61% of
patients continued using the first bDMARD. The leading cause of drug discontinuation
was inadequate response. Non-mycobacterium infection was the most common
SAE.
Financial support:
This study was supported by the Thai Rheumatism
Association.
Figure.
Drug
survival rate for biological disease modifying antirheumatic drugs
To cite this abstract in AMA style:
Narongroeknawin P, Katchamart W, Suwannalai P, Kasitanon N, Kitumnuaypong T, Mahakkanukrauh A, Siripaitoon B. Drug Survival and Reasons for Discontinuation of Biological Disease Modifying Antirheumatic Drug in Thai Patients with Rheumatoid Arthritis: Analysis from the Thai Rheumatic Disease Prior Authorization (RDPA) Register [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/drug-survival-and-reasons-for-discontinuation-of-biological-disease-modifying-antirheumatic-drug-in-thai-patients-with-rheumatoid-arthritis-analysis-from-the-thai-rheumatic-disease-prior-authorizatio/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-survival-and-reasons-for-discontinuation-of-biological-disease-modifying-antirheumatic-drug-in-thai-patients-with-rheumatoid-arthritis-analysis-from-the-thai-rheumatic-disease-prior-authorizatio/