Session Information
Date: Monday, November 9, 2015
Title: Health Services Research II: Rheumatoid Arthritis Treatment and Healthcare Utilization
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
Anti-TNF-α drugs are effective treatments for patients with
inflammatory arthritis (IA). They are however expensive and their use carries a
significant cost burden to the payer. Anti-TNF-α dose reduction in patients with stable disease in
remission could lead to significant cost savings.
The aim of this prospective, non-blinded, non-randomised, observational study was to observe whether
patients with IA (rheumatoid arthritis (RA), ankylosing
spondylitis (AS) and psoriatic arthritis (PsA)) could
successfully dose reduce anti-TNF-α over a 4 year period and to estimate the total cost
savings associated with dose reduction.
Methods:
Anti-TNF-α dose reduction was offered to patients with IA who
were in remission as defined by standardized disease activity indices (DAS-28
<2.6, BASDAI < 4). Patients
on etanercept were reduced from 50mg weekly to 50mg
fortnightly. Patients on adalimumab were reduced from
40mg fortnightly to 40mg monthly. Patients who agreed to dose reduction were
invited to participate in the study which commenced in
2010. Patients were assessed for disease activity at 3, 6 ,
12, 24, 36 and 48 months. Patients who remained in remission were encouraged to
stay on the reduced dose anti-TNF-α. The primary end-point was the number of patients
remaining on reduced dose anti-TNF-α at 4 years. Cost savings were estimated by deducting
the actual total cost of anti-TNF-α used from the theoretical cost of using full dose anti-TNF-α had dose reduction
not occurred.
Results:
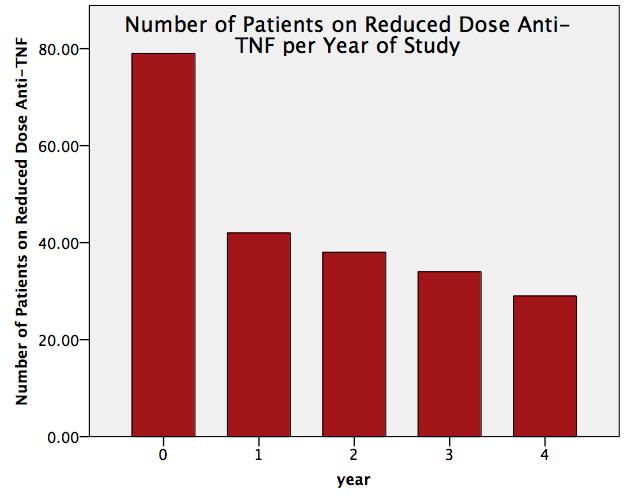
79 patients with IA in remission were recruited. 57% had RA
(n=45) ,13%
PsA (n= 10) and 30% AS (n= 24). 57% (n=45)
were on etanercept and 43% (n=34) were on adalimumab. The percentage of patients who remained on
reduced dose anti-TNF-α
at 4 years was 36% (n=29) (See figure 1). This resulted in net estimated savings
to the exchequer of $1,373,761. The average cost saving per patient included
in the study per year was $4346. A majority (53% n=37) of dose reduction
failures occurred within the first year . Of the
patients who successfully dose reduced at year 1 (n=42), a majority (69%, n=29) were
able to maintain the dose reduction up to year 4. A greater percentage of AS
patients (52 % n=12) were able to maintain dose
reduction up to year 4 but this was not significant.
Conclusion:
Dose reduction of anti-TNF-α therapy in patients with IA in remission is feasible
and can yield significant cost savings. Further studies could be designed to
help define which patients are more likely to successfully dose reduce.
To cite this abstract in AMA style:
Stack J, Murphy CL, Bannon C, Murphy E, Duffy T, Barry M. Drug Survival and Cost Effectiveness in Patients on Reduced Dose Anti-TNF: Results of a 4 Year Prospective Observational Study [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/drug-survival-and-cost-effectiveness-in-patients-on-reduced-dose-anti-tnf-results-of-a-4-year-prospective-observational-study/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/drug-survival-and-cost-effectiveness-in-patients-on-reduced-dose-anti-tnf-results-of-a-4-year-prospective-observational-study/